Abstract
Aims: We aimed to assess the impact of implant depth on hydrodynamic function following valve-in-valve (VIV) transcatheter aortic valve replacement (TAVR) using the ACURATE neo transcatheter heart valve (THV) through an ex vivo bench study.
Methods and results: Multiple implantation depths were tested at incremental depths of 2 mm using a small size ACURATE neo valve for VIV TAVR in 19 mm, 21 mm, 23 mm, and 25 mm Mitroflow bioprosthetic valves. Multimodality imaging and hydrodynamic evaluation was performed at each implantation depth. A low implantation was associated with higher transvalvular gradients. The highest transvalvular gradient was observed at −10 mm depth for 19 mm (40.0±0.5 mmHg), −8 mm for 21 mm (15.3±0.2 mmHg), −6 mm for 23 mm (14.7±0.3 mmHg) and −8 mm for 25 mm (8.4±0.2 mmHg) surgical valves. The lowest transvalvular gradient was observed at 0 mm depth for the 19 mm (14.9±0.2 mmHg)/21 mm (7.2±0.1 mmHg), and +2 mm depth for the 23 mm (5.7±0.1 mmHg)/25 mm (5.8±0.1 mmHg) surgical valves. At low implantation depth, there was worse leaflet pin-wheeling and also evidence of interaction of THV leaflets with those of the surgical valve that impaired leaflet coaptation, resulting in a high regurgitant fraction (42.5% in the 21 mm and 83.3% in the 23 mm surgical valve at −10 mm depths).
Conclusions: A high implant is desirable to facilitate favourable hydrodynamic function when performing VIV TAVR using the ACURATE neo THV for Mitroflow aortic bioprostheses sized ±25 mm. In a 19 mm Mitroflow valve, positioning the upper crown of the ACURATE neo THV above the posts of the surgical valve is desirable to facilitate favourable transvalvular gradients. Low implantation results in higher transvalvular gradients and worse pin-wheeling, and THV leaflet dysfunction can be severe due to interaction with the surgical valve.
Abbreviations
| TAVR | transcatheter aortic valve replacement |
| THV | transcatheter heart valve |
| VIV | valve-in-valve |
Introduction
Valve-in-valve (VIV) transcatheter aortic valve replacement (TAVR) is an alternative treatment to reoperation for patients with failed bioprosthetic surgical valves1,2. Different transcatheter heart valves (THV) can be utilised for VIV TAVR3. The ACURATE neo™ (Boston Scientific, Marlborough, MA, USA) is a commercially available THV4,5. However, there is less experience using this THV for VIV TAVR compared to other commercially available THV. The optimum position of this THV relative to the surgical valve is poorly understood.
We assessed the impact of implant depth on hydrodynamic function using the ACURATE neo THV for VIV TAVR in Mitroflow (Sorin Group Canada Inc., Burnaby, BC, Canada) aortic bioprosthetic valves.
Methods
VALVES
The surgical aortic valves tested were 19 mm, 21 mm, 23 mm and 25 mm Mitroflow aortic bioprostheses. One valve of each size was tested. The Mitroflow bioprosthesis consists of an acetyl homo-polymer stent frame with bovine pericardial sheets sutured externally to form the leaflets. The sewing ring covers the base of the frame and incorporates a non-rigid radiopaque silicone ring covered by a Dacron mesh6. The 19 mm, 21 mm, 23 mm and 25 mm Mitroflow valves have a true internal diameter of 15.5 mm, 17 mm, 19 mm and 21 mm, respectively7.
VIV TAVR was tested with the small ACURATE neo THV. The ACURATE neo is a self-expanding THV with a nitinol frame and porcine pericardial leaflets positioned high within the frame. There are inner and outer pericardial seals at the inflow level of the valve. There are three stabilisation arches for axial alignment, an upper crown and a lower crown (Figure 1A). The bases of the three stabilisation arches form three THV posts. The lower crown of a small ACURATE neo is composed of 15 diamond-shaped segments, of which three are taller in height and extend lower than the other 12 segments (Figure 1A). The total height of the ACURATE neo ranges between 48 mm and 51 mm with the stent body height being 18-19 mm. Three sizes (small, medium and large) are currently available to accommodate an aortic annulus diameter between 21 mm and 27 mm. The small size ACURATE neo was used in this study. The small size ACURATE neo accommodates an aortic annulus diameter between 21 mm and 23 mm, and a perimeter between 66 mm and 72 mm.
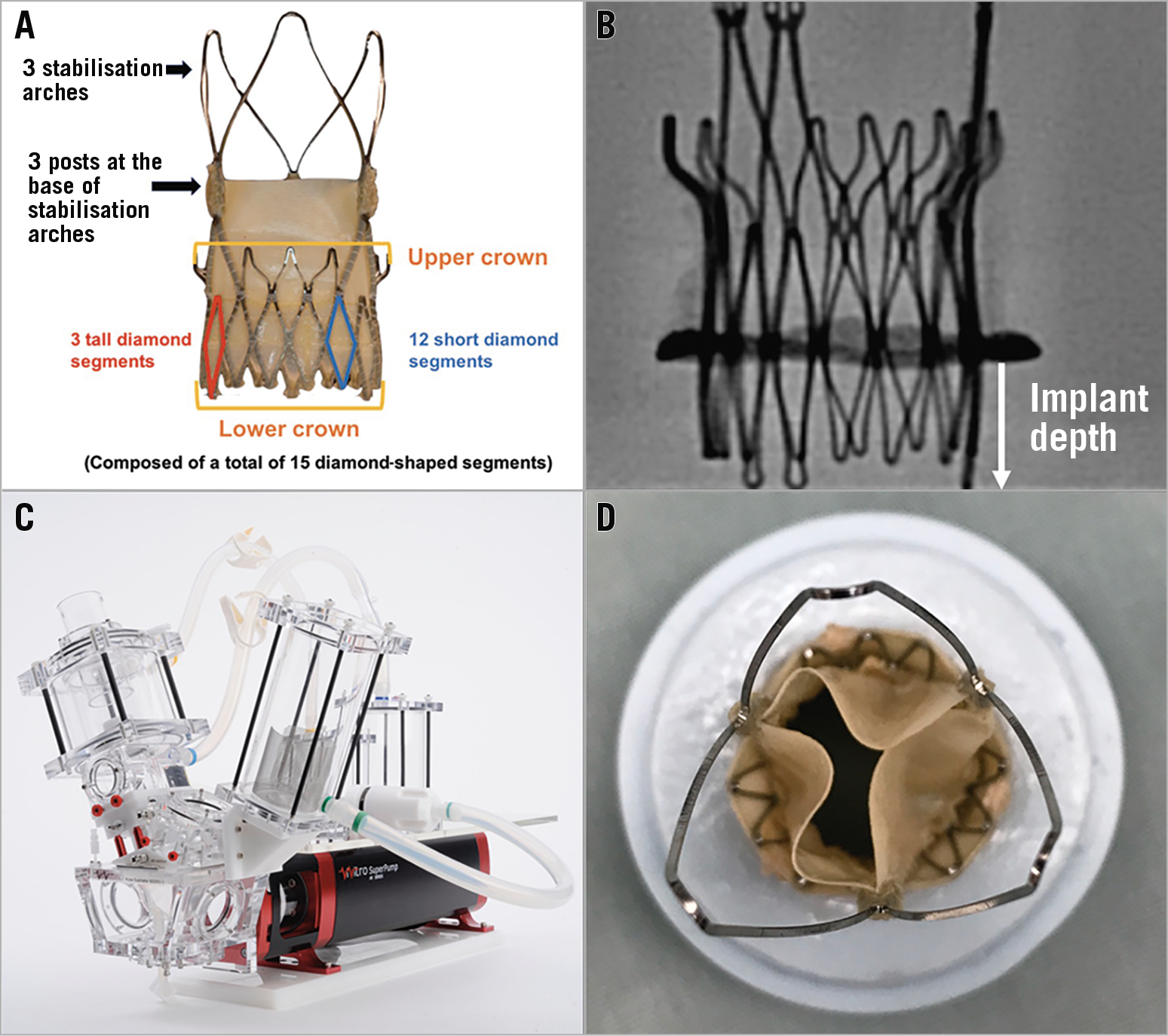
Figure 1. Ex vivo bench testing methodology. A) Example of an ACURATE neo THV. There are three stabilisation arches for axial alignment, an upper crown and a lower crown. The bases of the three stabilisation arches form three THV posts. The base of the upper crown is at the same level as the base of THV leaflet insertion. The lower crown of a small ACURATE neo is composed of 15 diamond-shaped segments, of which three are taller in height and extend lower than the other 12 segments. B) Implantation depth was measured from the lower border of the radiopaque ring of the Mitroflow valve to the lowest point of the lower crown (bottom of one of the tall diamond segments) of the ACURATE neo. C) Pulse duplicator used for hydrodynamic testing. D) Example of ex vivo VIV TAVR in silicone holder.
EX VIVO VALVE-IN-VALVE PROCEDURE
Multiple implantation depths were tested using a small size ACURATE neo valve for ex vivo VIV TAVR in the four sizes of Mitroflow tested. Implantation depth was measured from the lower border of the radiopaque ring of the Mitroflow valve to the lowest point of the lower crown (bottom of one of the tall diamond segments) of the ACURATE neo (Figure 1B). The radiopaque ring lies just below the plastic ring of the Mitroflow valve. Implantation depth was measured with both fluoroscopy and macroscopic measurements using digital scientific callipers. Implantation was tested at incremental depths of 2 mm from the minimum depth required to allow full expansion of the upper crown, to a maximum depth of 10 mm below the radiopaque ring of the Mitroflow valve. For the 19 mm and 21 mm Mitroflow, implantation depths of 0 mm, −2 mm, −4 mm, −6 mm, −8 mm and −10 mm were tested. For the 23 mm and 25 mm Mitroflow, implantation depths of +2 mm, 0 mm, −2 mm, −4 mm, −6 mm, −8 mm and −10 mm were tested.
IMAGING
Multimodality imaging was performed at each tested implant depth. High-resolution photography was performed at the same magnification and same fixed camera height. Fluoroscopy was performed using a standard adult cardiac catheterisation laboratory (General Electric Healthcare, Chicago, IL, USA).
HYDRODYNAMIC ASSESSMENT
Hydrodynamic testing was performed at each implant depth tested, using a commercially available pulse duplicator (ViVitro Labs Inc, Victoria, BC, Canada) (Figure 1C). Valves were tested in accordance with ISO 5840-3:2013 guidelines for in vitro pulsatile flow testing for heart valve substitutes implanted by transcatheter techniques8. Valves were placed in a holder fabricated from silicone with a durometer of scale Shore A hardness of 40±5 (Figure 1D). Justification for the selection of sample holder hardness was based on published data on acceptable tissue compliance matched with published data on the silicone material hardness scale9,10,11. The test fluid used was 0.9±0.2% sodium chloride test solution maintained at 37±2°C (one drop of Cosmocil® [preservative] per 1 L).
Valves were tested on the aortic side of the pulse duplicator with a spring-loaded disc valve (ViVitro Labs) on the mitral side of the pulse duplicator. Measurements were based on average results taken from 10 consecutive cycles. A high-speed moving image was captured at each step condition. High-speed moving images were used to assess for leaflet pin-wheeling. Pin-wheeling, as defined by the International Standards Organization guideline for THV testing, refers to twisting of the leaflet free edges resulting from excessive leaflet redundancy8. Pulsatile forward flow performance was tested at a nominal beat rate of 70±1 beats per minute, systolic duration of 35±5%, mean aortic pressure of 100±2 mmHg, and simulated cardiac output of 5±0.1 litres per minute. Mean gradient (mmHg), regurgitant fraction (%) and effective orifice area (cm2) were assessed.
Results
The upper crown of the THV was above the posts of the Mitroflow valve at an implant depth of 0 mm for the 19 mm/21 mm and +2 mm for the 23 mm/25 mm Mitroflow valves. In the 19 mm Mitroflow there was significant underexpansion of the lower crown of the THV. With lower implantation depths, there was evidence of underexpansion of the upper crown of the THV (Figure 2).
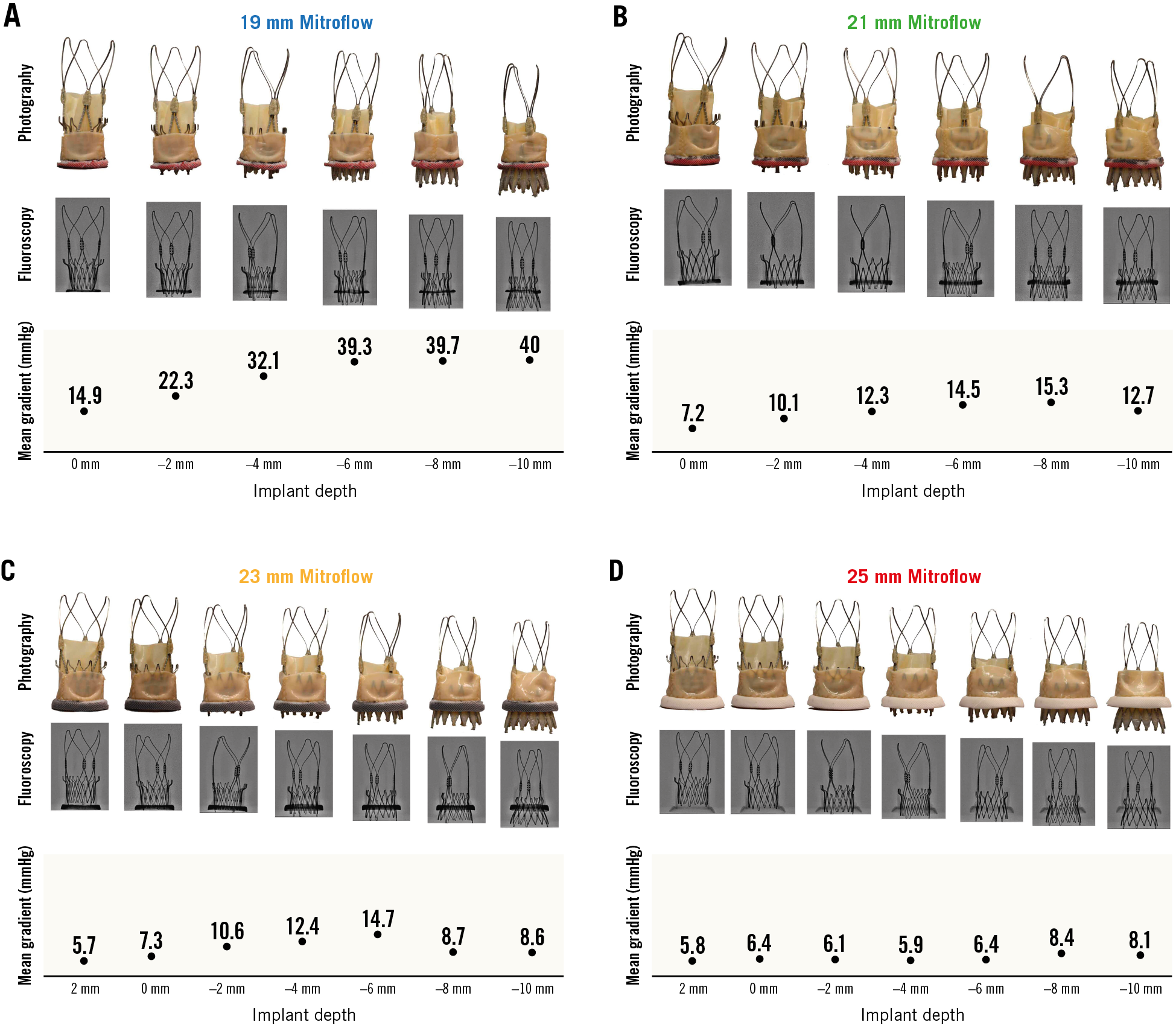
Figure 2. Photography, fluoroscopy and mean gradient by implant depth for VIV TAVR with the ACURATE neo in Mitroflow bioprosthetic valves. A) VIV TAVR with ACURATE neo in 19 mm Mitroflow bioprosthetic valve. B) VIV TAVR with ACURATE neo in 21 mm Mitroflow bioprosthetic valve. C) VIV TAVR with ACURATE neo in 23 mm Mitroflow bioprosthetic valve. D) VIV TAVR with ACURATE neo in 25 mm Mitroflow bioprosthetic valve.
VALVE HYDRODYNAMICS
MEAN GRADIENT
In the 19 mm Mitroflow following VIV TAVR, transvalvular gradients were >20 mmHg for implant depths between −2 mm and −10 mm. The lowest gradient observed in the 19 mm Mitroflow VIV was 14.9±0.2 mmHg at an implant depth of 0 mm (Moving image 1) and the highest gradient was 40.0±0.5 mmHg at an implant depth of −10 mm (Figure 2A, Moving image 2). In the 21 mm Mitroflow following VIV TAVR, transvalvular gradients were <20 mmHg for all implant depths tested. The lowest gradient observed in the 21 mm Mitroflow was 7.2±0.1 mmHg at an implant depth of 0 mm (Moving image 3) and the highest gradient was 15.3±0.2 mmHg at an implant depth of −8 mm (Figure 2B, Moving image 4). In the 23 mm Mitroflow following VIV TAVR, transvalvular gradients were <20 mmHg for all implant depths tested. The lowest gradient observed in the 23 mm Mitroflow was 5.7±0.1 mmHg at an implant depth of +2 mm (Moving image 5) and the highest gradient was 14.7±0.3 mmHg at an implant depth of −6 mm (Figure 2C, Moving image 6). In the 25 mm Mitroflow following VIV TAVR, transvalvular gradients were <10 mmHg for all implant depths tested. The lowest gradient observed in the 25 mm Mitroflow was 5.8±0.1 mmHg at an implant depth of +2 mm (Moving image 7) and the highest gradient was 8.4±0.2 mmHg at an implant depth of −8 mm (Figure 2D, Moving image 8).
EFFECTIVE ORIFICE AREA (EOA)
Effective orifice areas for each implant depth for the four valves tested are detailed in Figure 3. The largest EOA observed for the 19 mm Mitroflow following VIV TAVR was 1.6 cm2 at an implant depth of 0 mm, and the smallest EOA was 1.0 cm2 at an implant depth between −6 mm and −10 mm. The largest EOA observed for the 21 mm Mitroflow following VIV TAVR was 2.4 cm2 at an implant depth of 0 mm, and the smallest EOA was 1.6 cm2 at an implant depth of −8 mm. The largest EOA observed for the 23 mm Mitroflow following VIV TAVR was 2.7 cm2 at an implant depth of +2 mm, and the smallest EOA was 1.6 cm2 at an implant depth of −6 mm. The largest EOA observed for the 25 mm Mitroflow following VIV TAVR was 2.8 cm2 at an implant depth of +2 mm, and the smallest EOA was 2.2 cm2 at an implant depth of −8 to −10 mm.
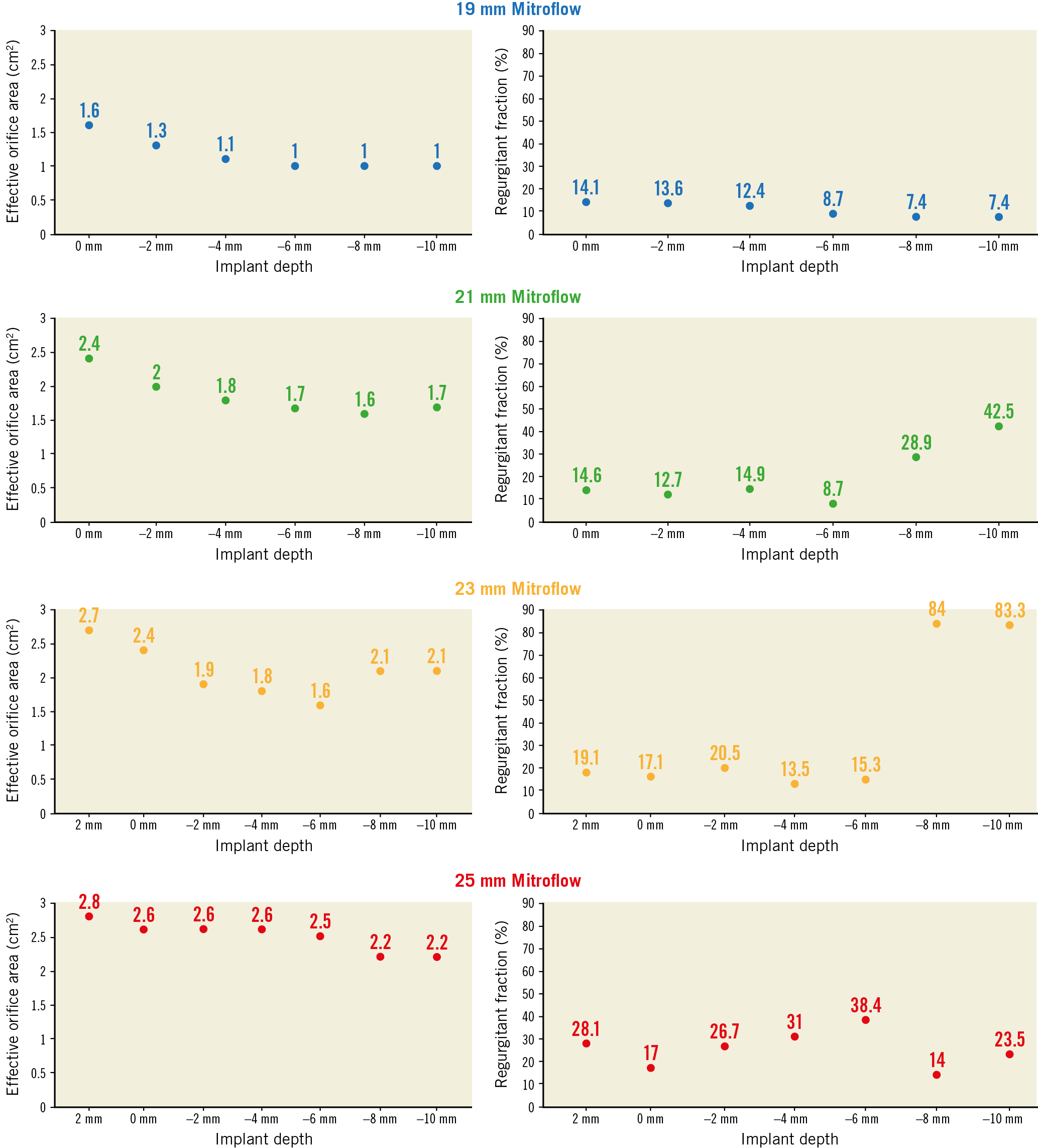
Figure 3. Effective orifice area and regurgitant fraction by implant depth for VIV TAVR with the ACURATE neo in Mitroflow bioprosthetic valves.
REGURGITANT FRACTION
Regurgitant fractions (%) at different implant depths in the four Mitroflow valves tested are outlined in Figure 3. At an implantation depth of −8 mm and −10 mm for the 21 mm and 23 mm Mitroflow valves, there was significant increase in regurgitant fraction (21 mm: 84% at −8 mm and 83.3% at −10 mm; 23 mm: 28.9% at −8 mm and 42.5% at −10 mm). In the 21 mm and 23 mm Mitroflow valves, the posts of the THV were not aligned with the posts of the Mitroflow, and at an implantation depth of −8 to −10 mm there was interaction of THV leaflets with Mitroflow leaflets which impaired leaflet closure (Figure 4, Figure 5). There was also evidence of cycle to cycle variation with leaflet interaction (Moving image 4, Moving image 6). In the 19 mm and 25 mm Mitroflow valves, the posts of the THV were aligned with the posts of the Mitroflow valve, and there was no observed interaction of the THV leaflets with the surgical valve leaflets at lower implant depths. For the 25 mm Mitroflow valve at an implant depth of −8 mm and −10 mm, the THV valve leaflets coapted below the level of the surgical valve leaflets (Figure 4, Figure 5, Moving image 8).
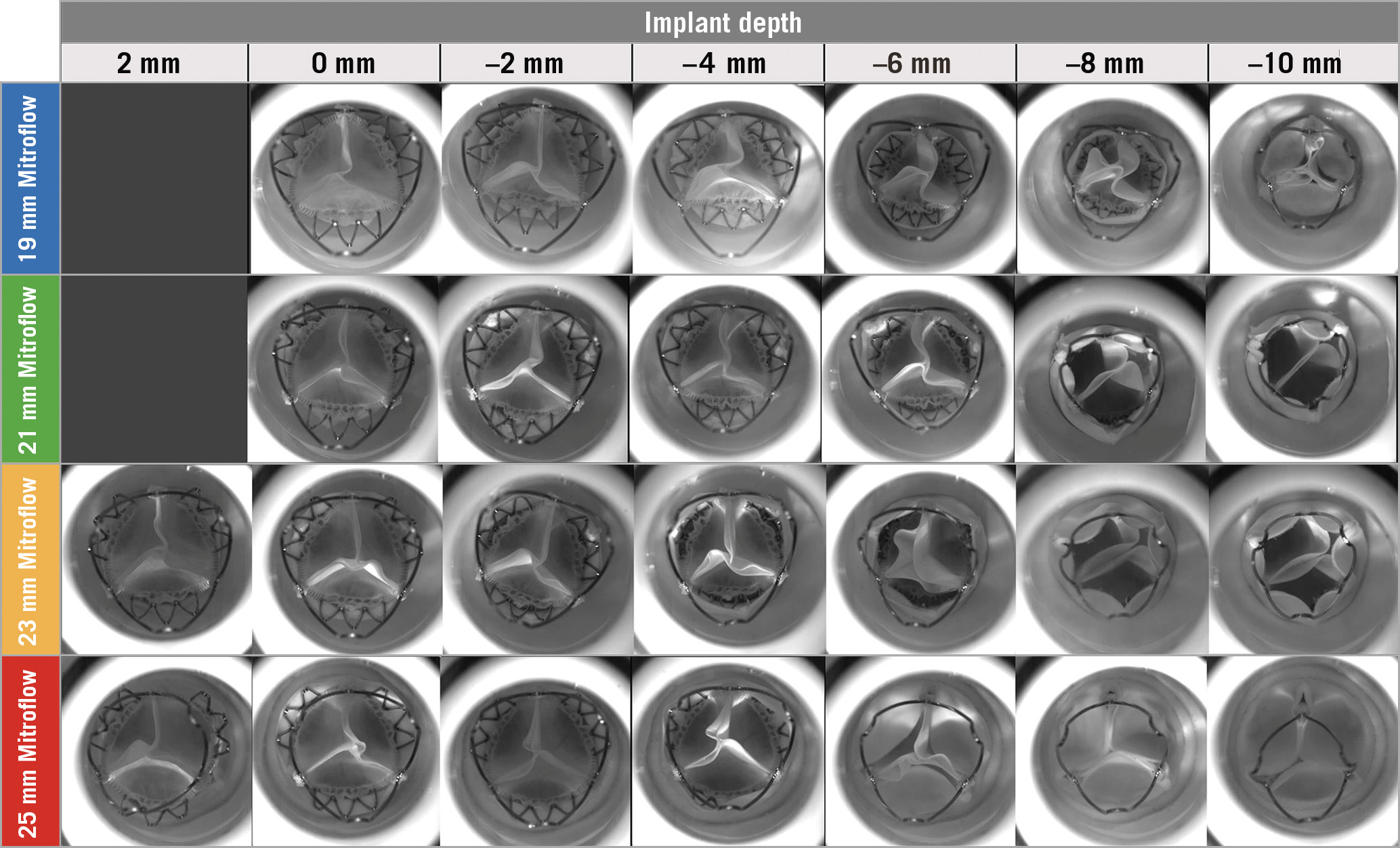
Figure 4. Images by implant depth for VIV TAVR with the ACURATE neo in Mitroflow bioprosthetic valve with backward pressure.
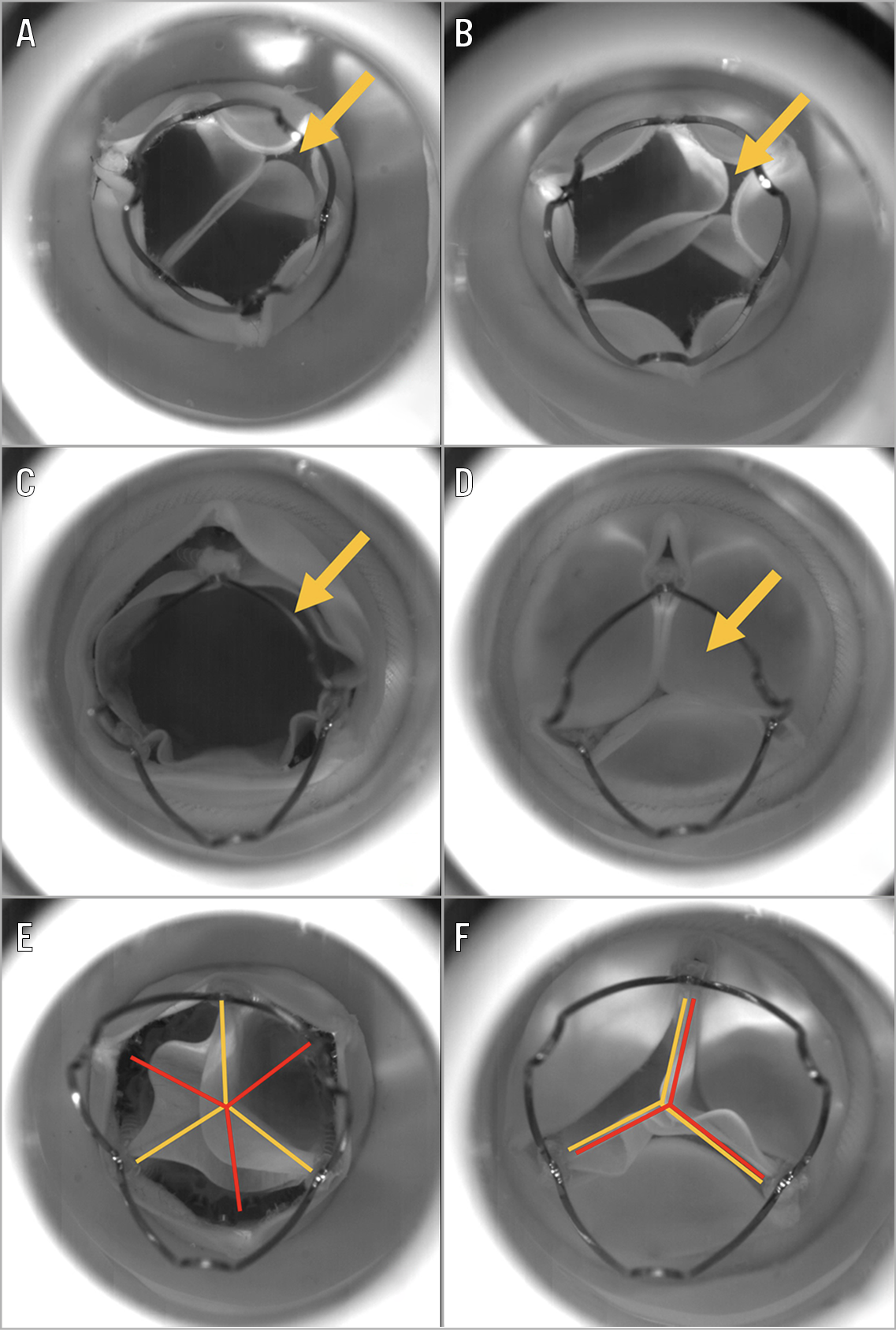
Figure 5. Images demonstrating THV leaflet interaction with the surgical valve leaflets. A) Image of VIV TAVR with 21 mm Mitroflow and small ACURATE neo at an implant depth of −10 mm. Arrow indicates interaction of the leaflet with the surgical valve leaflet preventing full closure and coaptation of this leaflet. B) Image of VIV TAVR with 23 mm Mitroflow and small ACURATE neo at an implant depth of −10 mm. Arrow indicates interaction of the leaflet with the surgical valve leaflet preventing full closure and coaptation of this leaflet. C) Image of VIV TAVR with 25 mm Mitroflow and small ACURATE neo at an implant depth of −10 mm. Arrow indicates that the THV leaflets are below the level of the surgical valve leaflets. D) Image of VIV TAVR with 25 mm Mitroflow and small ACURATE neo at an implant depth of −10 mm. Arrow shows that with backward pressure the surgical valve leaflets coapt above the THV valve leaflet level. E) Image of VIV TAVR with 23 mm Mitroflow and small ACURATE neo at an implant depth of −6 mm. The orange line indicates the position of the posts of the ACURATE neo. The red line indicates the position of the posts of the Mitroflow valve. The posts of the THV and surgical valve are not in alignment. Interaction of the THV leaflets with the surgical valve leaflets was observed. F) Image of VIV TAVR with 25 mm Mitroflow and small ACURATE neo at an implant depth of −6 mm. The orange line indicates the position of the posts of the ACURATE neo. The red line indicates the position of the posts of the Mitroflow valve. The posts of the THV and surgical valve are in alignment. No interaction of the THV leaflets with the surgical valve leaflets was observed.
PIN-WHEELING
With lower implantation depths, progressively worsening leaflet pin-wheeling was observed (Figure 4, Moving image 1, Moving image 3, Moving image 5, Moving image 7). It was not possible to comment on the degree of pin-wheeling at implantation depths of −8 and −10 for the 21 mm Mitroflow and −6 to −10 for the 23 mm Mitroflow, due to interaction of the THV leaflets with those of the surgical valve leaflets. In smaller valves, at lower implant depths there was evidence of impaired leaflet opening on forward pressure with bending and infolding of THV leaflets (Figure 6, Moving image 2, Moving image 4, Moving image 6, Moving image 8).
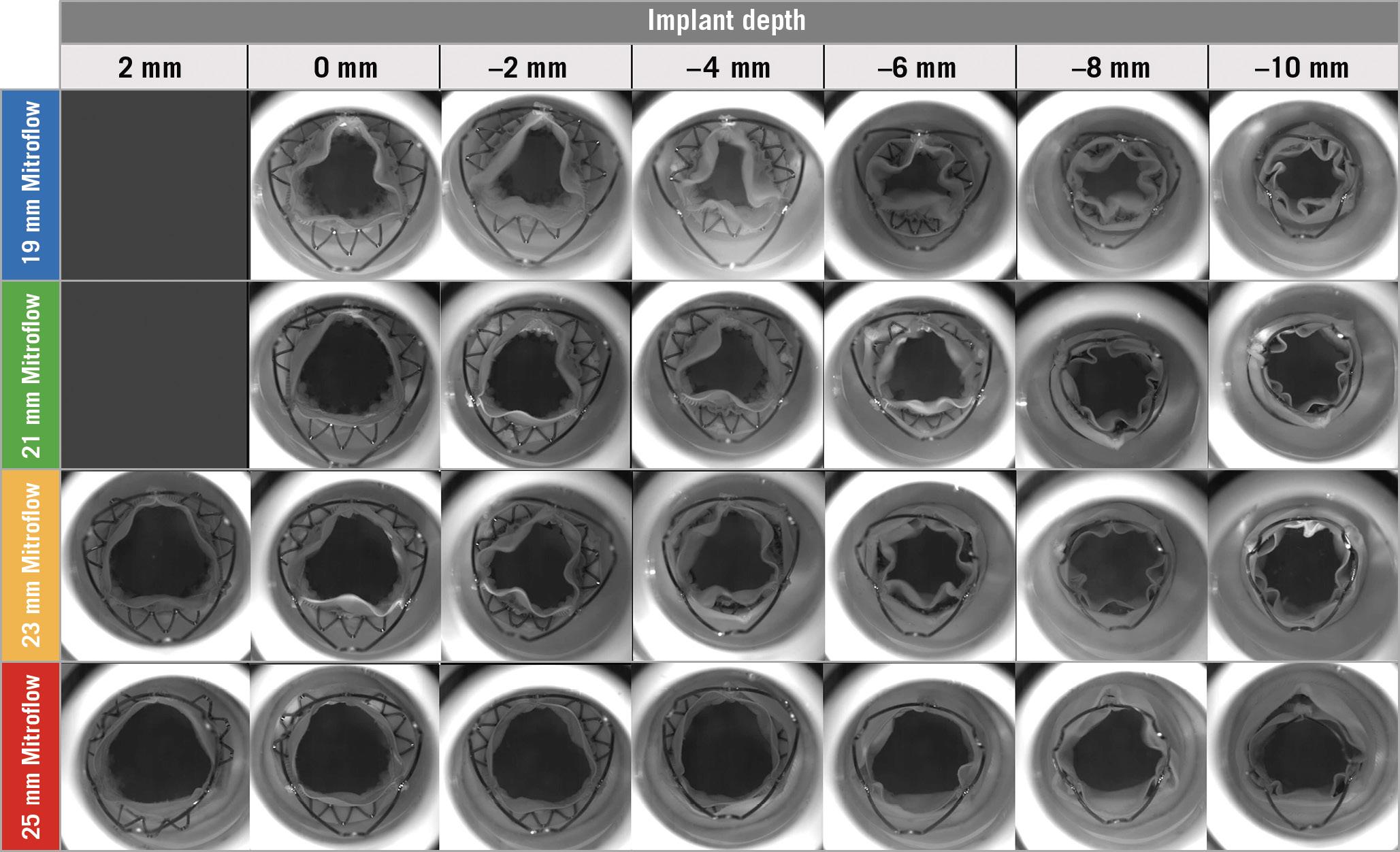
Figure 6. Images by implant depth for VIV TAVR with the ACURATE neo in Mitroflow bioprosthetic valve with forward pressure.
Discussion
When performing VIV TAVR using the small ACURATE neo THV in Mitroflow valves ≤25 mm, a high implant is desirable to facilitate lower transvalvular gradients, larger effective orifice area and low regurgitant fraction. In the 19 mm Mitroflow, positioning the upper crown of the THV above the posts of the Mitroflow bioprosthetic valve resulted in the lowest transvalvular gradient. Avoidance of a low implantation depth reduces the risk of leaflet pin-wheeling and interaction of THV leaflets with those of the surgical valve.
For VIV TAVR, achieving a THV position that facilitates maximal expansion and a supra-annular leaflet position is considered desirable to allow optimal leaflet coaptation and durability, and reduce the risk of patient-prosthesis mismatch2,3,12. Some THVs by design have leaflets that are positioned high within the frame. Any THV, if positioned so that the THV leaflets are above the surgical valve, will achieve a supra-annular position. In smaller surgical valves, high residual gradients following VIV TAVR remain a challenge, and a supra-annular leaflet position may achieve favourable hydrodynamic function following VIV TAVR1,2,3,13,14,15. In the case of the ACURATE neo, the position of the upper crown is of importance when determining implantation depth, particularly for VIV TAVR in a 19 mm Mitroflow. The upper crown of the THV needs to be fully expanded to allow optimal leaflet coaptation and function. If the upper crown is deployed within a 19 mm Mitroflow, there is significant underexpansion which is associated with high transvalvular gradients and leaflet pin-wheeling that may impact on long-term durability. Achieving a THV position where the upper crown lies just above the posts of the Mitroflow allows better expansion and superior hydrodynamic function, particularly for a 19 mm Mitroflow. However, achieving this position in a degenerated surgical valve, particularly one with poor radiopaque markers and with a THV that is non-recapturable, may be challenging. There may also be less ability to control implant depth, when the THV is deployed under physiological conditions. A high implantation, while desirable, must be balanced against the risk of THV embolisation. Performance of VIV TAVR using a THV design with limited clinical experience must be approached with caution. There is currently less experience with the ACURATE neo THV compared to other THV designs. There are currently no VIV TAVR bench studies or clinical series comparing the ACURATE neo THV to other THV designs for the Mitroflow bioprosthetic valve.
The commonly used aortic VIV app, available for download on smartphones, only recommends VIV TAVR using the small ACURATE neo for Mitroflow valves sized 25 mm and 27 mm. This recommendation, as stated in the app, is based on manufacturer guidance due to concerns that the THV will not be fully expanded in Mitroflow valves <25 mm. If an ACURATE neo valve is chosen, an implant depth of 15-20% is recommended by the app7. In our bench study, we demonstrated that VIV TAVR can be performed with favourable transvalvular gradients in both the 21 mm and 23 mm Mitroflow valves, with superior hydrodynamic function achieved with a higher implant. While transvalvular gradients were still within acceptable clinical limits with lower implantation, there was risk of severe leaflet dysfunction. In the Mitroflow valves sized 21 mm to 25 mm, there was a potential risk of high regurgitant fraction and leaflet pin-wheeling. With very low implantation, the THV leaflets could even be implanted below the level of the surgical valve leaflets. Factors that may influence regurgitant fraction are implant depth and orientation of the ACURATE neo THV to the posts of the Mitroflow valve. If the posts of the THV were misaligned relative to the Mitroflow valve posts, there was interaction of the THV leaflets with the Mitroflow valve that impacted on leaflet closure and was associated with high regurgitant fraction. Clinically, it would be very challenging to control the orientation of the THV posts, but leaflet interaction can be avoided by implanting the THV higher so that the THV leaflets are above the level of the surgical valve. Alternatively, use of a THV with an alternative design where the leaflets are surrounded by a stent frame would prevent any interaction of THV leaflets with those of the surgical valve. Significant pin-wheeling was also observed with lower implantation depths. Pin-wheeling can lead to localised leaflet strain that may accelerate leaflet fatigue and premature THV failure. Pin-wheeling as demonstrated on ex vivo testing has been shown to cause asymmetrical leaflet strain in THVs12,16,17. While our bench study demonstrates favourable hydrodynamic function with a high implant, clinical experience using the ACURATE neo THV for VIV TAVR remains limited, and the long-term durability and failure mechanisms of VIV TAVR using the ACURATE neo THV are unknown.
Following VIV TAVR in a Mitroflow valve, residual gradients may be elevated, particularly in small-sized surgical valves. In this situation, hydrodynamic function could be further optimised using techniques which modify the surgical valve such as bioprosthetic valve fracture. This technique involves fracturing the bioprosthetic surgical valve using non-compliant balloons and can be performed before or after VIV TAVR18,19. However, clinical experience using bioprosthetic valve fracture with the ACURATE neo THV is limited and the long-term implications of this technique are currently unknown.
Limitations
Ex vivo bench testing may not entirely reflect how the THV will expand in a patient’s native annulus, within a degenerated surgical bioprosthesis, or valve deployment under physiological conditions. It would be important to ascertain long-term durability with different implant depths. Only the Mitroflow valve was tested in this bench study, and one sample was tested for each size of bioprosthetic valve. While only one sample was used for each Mitroflow valve, hydrodynamic testing at each implant depth was based on 10 consecutive cycles, as specified by the International Standards Organization. It would also be desirable to assess other bioprosthetic surgical valve designs. Different surgical valve designs could influence THV hydrodynamic function.
Conclusions
A high THV implantation is desirable to facilitate favourable hydrodynamic function when performing valve-in-valve transcatheter aortic valve replacement using the ACURATE neo THV for Mitroflow aortic bioprostheses sized ≤25 mm. In addition to implant depth, a recognition of the position of the upper crown is of importance and influences hydrodynamic function, particularly in smaller surgical valves. Low implantation results in higher transvalvular gradients, and leaflet dysfunction can be severe.
|
Impact on daily practice A high implant is desirable to facilitate favourable hydrodynamic function when performing VIV TAVR using the ACURATE neo THV for Mitroflow aortic bioprostheses sized ≤25 mm. In a 19 mm Mitroflow valve, positioning the upper crown of the ACURATE neo THV above the posts of the surgical valve is desirable to facilitate favourable transvalvular gradients. A low implantation depth carries risks of high residual gradients, particularly in a 19 mm Mitroflow, that may be associated with poor long-term clinical outcomes. Importantly, in larger-sized Mitroflow valves, while residual gradient may be acceptable, avoidance of a low implantation depth also reduces the risk of leaflet pin-wheeling and interaction of the THV leaflets with those of the surgical valves. This THV leaflet interaction can lead to severe leaflet dysfunction and may impact on durability. |
Conflict of interest statement
P. Blanke is a consultant to Edwards Lifesciences. D. Dvir is a consultant to Edwards Lifesciences, Medtronic and St. Jude Medical. J. Leipsic is a consultant to Edwards Lifesciences and provides CT core lab services for Edwards Lifesciences, Medtronic, Neovasc, GDS, and Tendyne Holdings, for which no direct compensation is received. J. Leipsic has stock options in, is a consultant to, and receives institutional research support from HeartFlow. J. Leipsic is supported by a Canadian Research Chair in Advanced CardioPulmonary Imaging. P. Pibarot has received funding from Edwards Lifesciences for echocardiography core lab analyses in the field of TAVR including valve-in-valve, with no direct personal compensation. S. Toggweiler is a consultant and proctor for Boston Scientific and NVT GmbH. He has also received an institutional research grant from Boston Scientific. J.G. Webb is a consultant to, and has received research funding from, Edwards Lifesciences, Abbott Vascular, and ViVitro Labs. D. Wood is a consultant to Edwards Lifesciences. J. Sathananthan has received speaking fees from Edwards Lifesciences. The other authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. High-speed moving image of VIV TAVR with 19 mm Mitroflow and small ACURATE neo at an implant depth of 0 mm.
Moving image 2. High-speed moving image of VIV TAVR with 19 mm Mitroflow and small ACURATE neo at an implant depth of −10 mm.
Moving image 3. High-speed moving image of VIV TAVR with 21 mm Mitroflow and small ACURATE neo at an implant depth of 0 mm.
Moving image 4. High-speed moving image of VIV TAVR with 21 mm Mitroflow and small ACURATE neo at an implant depth of −10 mm.
Moving image 5. High-speed moving image of VIV TAVR with 23 mm Mitroflow and small ACURATE neo at an implant depth of 0 mm.
Moving image 6. High-speed moving image of VIV TAVR with 23 mm Mitroflow and small ACURATE neo at an implant depth of −10 mm.
Moving image 7. High-speed moving image of VIV TAVR with 25 mm Mitroflow and small ACURATE neo at an implant depth of 0 mm.
Moving image 8. High-speed moving image of VIV TAVR with 25 mm Mitroflow and small ACURATE neo at an implant depth of −10 mm.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 7. High-speed moving image of VIV TAVR with 25 mm Mitroflow and small ACURATE neo at an implant depth of 0 mm.
Moving image 8. High-speed moving image of VIV TAVR with 25 mm Mitroflow and small ACURATE neo at an implant depth of <10 mm.
Moving image 1. High-speed moving image of VIV TAVR with 19 mm Mitroflow and small ACURATE neo at an implant depth of 0 mm.
Moving image 2. High-speed moving image of VIV TAVR with 19 mm Mitroflow and small ACURATE neo at an implant depth of <10 mm.
Moving image 3. High-speed moving image of VIV TAVR with 21 mm Mitroflow and small ACURATE neo at an implant depth of 0 mm.
Moving image 4. High-speed moving image of VIV TAVR with 21 mm Mitroflow and small ACURATE neo at an implant depth of <10 mm.
Moving image 5. High-speed moving image of VIV TAVR with 23 mm Mitroflow and small ACURATE neo at an implant depth of 0 mm.
Moving image 6. High-speed moving image of VIV TAVR with 23 mm Mitroflow and small ACURATE neo at an implant depth of <10 mm.

