
Abstract
Aims: The aim of this study was to determine the best functional position of a transcatheter heart valve (THV) implanted as a valve-in-valve (ViV) procedure in small rapid deployment valves (RDV) in an in vitro model.
Methods and results: A 21 mm Perceval, Enable or INTUITY RDV was mounted into a pulse duplicator and a 23 mm balloon-expandable or a self-expanding THV was deployed (valve-in-valve) in two different positions. Under physiological hydrodynamic conditions, the performance of the THV was characterised by mean transvalvular pressure gradient (MPG), effective orifice area (EOA) and regurgitation volume (RV). Leaflet kinematics were assessed with high-speed video recordings, and X-ray images were acquired. All THV/RDV combinations met ISO requirements regarding hydrodynamic performance. In most cases, the higher position of the THV performed better than the lower one in terms of a lower MPG and increased EOA. Leaflet motion of the implanted THV was impaired in the lower position. In contrast, regurgitation volumes were relatively small and similar, regardless of the THV position.
Conclusions: ViV implantation of a THV in a small RDV yielded satisfactory hydrodynamic results. In most cases, a high implantation position achieved lower MPG, higher EOA and a reduced risk of impaired THV leaflet function. Fluoroscopy images of the best functional ViV positions are presented as a blueprint for patient procedures.
Introduction
Aortic valve disease is the most common valvular heart disease in developed countries and its prevalence increases with age1. Surgical aortic valve replacement (SAVR) has been the standard of care for treatment of severe aortic stenosis for decades2,3. Nowadays, refined operative techniques allow less invasive approaches through a mini-sternotomy and thereby reduce the surgical trauma4-6. To shorten the cardiopulmonary bypass and cross-clamp time for the patient, so-called “rapid deployment valves” (RDV) (also known as “sutureless” valves) made of stented biological tissue have been introduced7. These valves do not require manual placement of sutures along the entire circumference of the aortic annulus and therefore facilitate the implantation procedure. In Europe, three devices have been approved for commercial use in the aortic position, the Perceval™ S (Sorin [now LivaNova], Sallugia, Italy), the 3f Enable™ (Medtronic, Minneapolis, MN, USA) and the EDWARDS INTUITY™ Elite Valve System (Edwards Lifesciences, Irvine, CA, USA). Very recently, the 3f Enable has been withdrawn from the European market by the company. The Perceval S, on the other hand, has been approved by the US FDA8. The three RDV differ significantly in terms of prosthesis design and implantation techniques and, similar to standard surgical bioprostheses, are probably prone to degeneration, in particular when implanted in younger patients.
For the treatment of degenerated surgical bioprostheses, transcatheter valve-in-valve (ViV) procedures are increasingly performed in patients exhibiting an elevated operative risk. They offer an alternative approach to redo SAVR in patients suffering from a degenerated RDV. However, insights into the ViV International Data Registry (VIVID) revealed an increased risk for device malposition, elevated pressure gradients and impaired haemodynamic function compared to standard TAVI9. In addition, to date there is only very little experience in the treatment of dysfunctional RDV. Redo SAVR after implantation of an RDV can be technically very demanding as a consequence of their particular stent design and tissue ingrowth, increasing the operative risk for the patient. Recently, our group has developed an in vitro algorithm to simulate a ViV procedure into a dysfunctional Perceval S prosthesis and determine the best implantation position of the THV. The acquired experimental data correlated very well with the in vivo performance and ensured a satisfactory patient outcome whilst minimising the procedural risk for the patient10. Finally, with the increasing use of bioprosthetic valves, particularly in younger patients, valve degeneration will be more frequently seen. It therefore seems necessary to develop a strategy in order to treat high-risk patients with degenerated or dysfunctional RDV in the future.
Therefore, the aim of the current study was to expand on our previous findings and assess the hydrodynamic performance of a balloon-expandable and a self-expanding THV when implanted into the three above-mentioned RDV. In particular, we sought to find the optimal RDV/THV combination and define the best functional implantation position for the respective THV, thereby providing crucial information for future ViV procedures into RDV.
Methods
CHOICE OF DEVICES
Based on our past experience, the choice of the THV and its implantation height seem to be particularly important for a successful ViV procedure10. In general, patients with small surgical bioprostheses have a higher risk of patient prosthesis mismatch and elevated transvalvular gradients following ViV procedures9, and therefore they represent the most challenging group of patients. Therefore, the smallest RDV available were used for this ViV experiment. For the ViV procedure, the two THV devices that are currently approved for this procedure were used.
RAPID DEPLOYMENT HEART VALVES
The Perceval S valve, size S, is a biological prosthesis composed of bovine pericardial cusps mounted on a super-elastic nitinol frame (Figure 1A, Figure 1B). Unique features include two nitinol rings, one at the inflow portion and one at the sinotubular junction, which are connected by straight commissural and sinusoidal struts which help to stabilise the prosthesis in its final position11. The 3f Enable prosthesis, 21 mm, is also composed of a nitinol stent with equine pericardial cusps (Figure 1C, Figure 1D). Similar to the Perceval S, the 3f Enable is characterised by a high valve profile, facilitating stabilisation of the prosthesis in vivo 12. The EDWARDS INTUITY Elite Valve System, 21 mm, is a bovine pericardial prosthesis with a cloth-covered stent frame at the inflow aspect13. Fixation within the annulus is achieved by balloon dilatation. There is no upper stent frame exceeding the dimensions of the commissural struts (Figure 1E, Figure 1F).
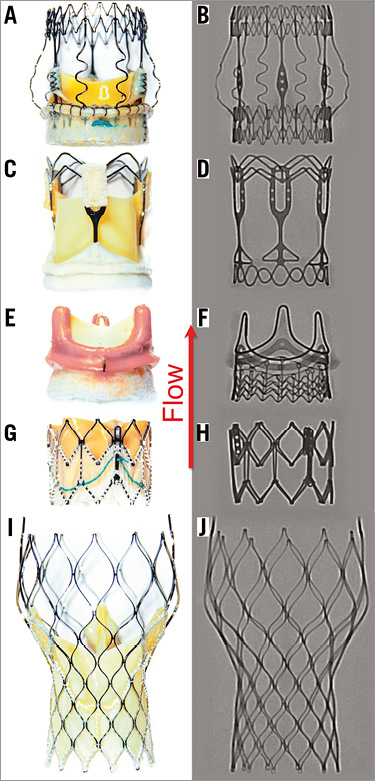
Figure 1. Ex vivo photographs and X-ray images of the three RDV and two THVs investigated. A) & B) Perceval S (size S). C) & D) 3f Enable (21 mm). E) & F) EDWARDS INTUITY (21 mm). G) & H) Edwards SAPIEN XT (23 mm). I) & J) CoreValve Evolut (23 mm). The red arrow indicates direction of flow.
TRANSCATHETER HEART VALVES
The Edwards SAPIEN XT™ (ESXT), 23 mm (Edwards Lifesciences) is a balloon-expandable, bovine pericardial bioprosthesis based on a cobalt-chromium stent frame14 (Figure 1G, Figure 1H). The CoreValve® Evolut™ (MCVE) 23 mm (Medtronic) is a self-expanding nitinol-framed valve with porcine pericardial cusps15 (Figure 1I, Figure 1J). Both devices are currently CE- and FDA-approved for the treatment of degenerated bioprostheses.
VALVE-IN-VALVE PROCEDURES
The RDV were placed within a custom-made silicone aortic annulus model of 21 mm diameter, which corresponds to the in vivo annulus size recommended by the manufacturers for the prosthesis sizes of the RDV used in this study. Each RDV was implanted into the aortic model according to the manufacturers’ instructions, employing the original delivery devices. Afterwards, both THVs were consecutively implanted into the RDV according to the manufacturers’ instructions. Two implantation positions were chosen for each THV/RDV combination: in the low implantation position, the lower margins of the THV (Figure 2, Figure 3, yellow marker) and RDV (Figure 2, Figure 3, blue marker) were aligned, or the lower THV margin exceeded the lower RDV margin by approximately 2 mm for better anchorage of the THV in the RDV annulus, whereas in the high position the lower margin of the THV was placed approximately 3-5 mm above the bottom margins of each RDV (Figure 2, Figure 3). In addition, each RDV was inspected in detail with regard to stent and valve design to ensure that both positions were feasible, stable and clinically relevant (Figure 2, Figure 3).
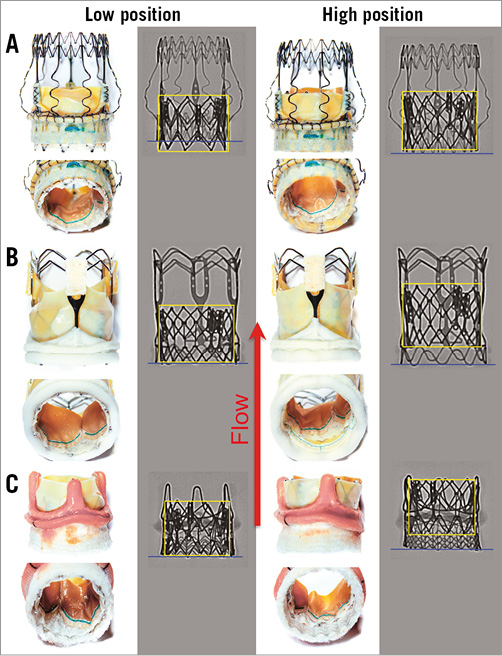
Figure 2. Ex vivo photographs and X-ray images of the ESXT implanted as a ViV procedure in the lower and higher position in the three RDV. A) Perceval S. B) 3f Enable. C) INTUITY. The yellow box indicates the positioning of the ESXT proportional to the lower margin of the respective RDV (blue line). The red arrow indicates direction of flow.
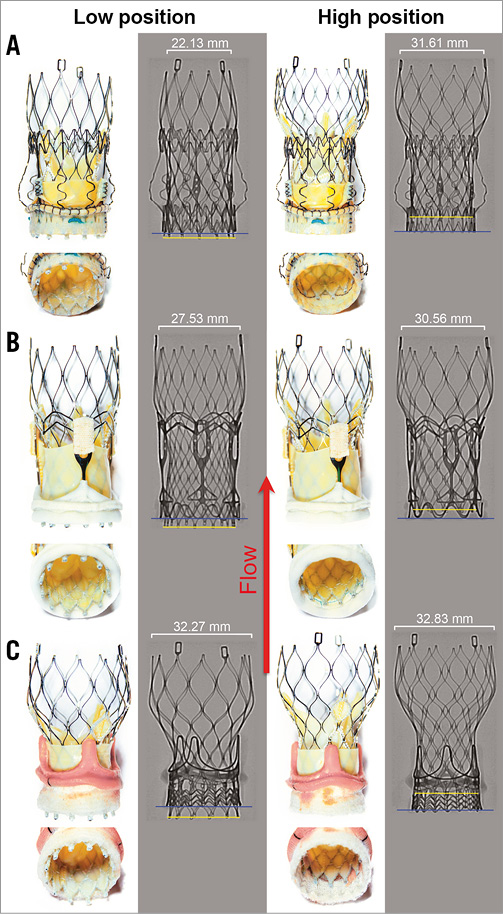
Figure 3. Ex vivo photographs and X-ray images of the MCVE implanted as a ViV procedure in the lower and higher position in the three RDV. A) Perceval S. B) 3f Enable. C) INTUITY. The yellow line indicates the lower margins of the MCVE proportional to the lower margin of the respective RDV (blue line). The red arrow indicates direction of flow.
HYDRODYNAMIC MEASUREMENTS
The silicone annuli containing the RDV were placed within the aortic valve compartment of the pulse duplicator developed at the Department of Cardiovascular Engineering CVE-AME at the Helmholtz Institute, Germany16. This system is designed to mimic flow conditions of the left heart and has been employed successfully in comparable studies10,17.
Hydrodynamic measurements were performed under three different conditions, simulating low cardiac output, light exercise and standard reference parameters, in accordance with ISO regulations (cardiac output 5 L/min, 70 heart beats per minute, mean aortic pressure 100 mmHg) with a 0.9% sodium chloride solution. Due to the clinical significance, only measurements under ISO conditions are shown. Data acquisition was run across ten consecutive cardiac cycles, and mean transvalvular pressure gradient (MPG), effective orifice area (EOA) and total regurgitation volume (RV) were determined. The RV is given as a percentage of the forward flow and was differentiated into closure and the leakage volume according to the ISO 5840-3. The closure volume results from regurgitation during the end of systole until closure of the aortic valve, whereas the leakage volume is defined as any regurgitation recorded after closure of the aortic valve. Each valve-in-valve combination was measured in a single experimental set-up, and each of the mean values from this single measurement of ten consecutive cardiac cycles was taken.
HIGH-SPEED CAMERA RECORDING
High-speed video recordings (FASTCAM 1024PCI, Model 100 K; Photron, San Diego, CA, USA) of valve performance during hydrodynamic testing were analysed in terms of leaflet opening and closing dynamics, leaflet coaptation and possible leaflet/stent-frame interaction.
X-RAY IMAGING
X-ray pictures (Artis zeego; Siemens, Erlangen, Germany) of all ViV positions were taken under direct visual control to have a pattern and guidance for future patient procedures.
STATISTICAL ANALYSIS
All data are given as mean±standard deviation. The difference in the hydrodynamic performance between different positions was assessed using Welch’s t-test. A p-value <0.05 was considered statistically significant.
Results
HYDRODYNAMIC MEASUREMENTS FOR VIV IMPLANTATION IN THE PERCEVAL S
The MPG within the 21 mm Perceval S prosthesis was similar for the 23 mm ESXT in both positions (low position 13.38±0.24 vs. high position 13.47±0.25 mmHg, p=n.s.) (Figure 2A, Figure 4A). In contrast, the 23 mm MCVE showed differences in MPG with respect to the implantation positions (11.62±0.25 vs. 12.16±0.24 mmHg, p<0.001) (Figure 3A, Figure 4A). Interestingly, there was no difference in EOA when the ESXT was implanted into the Perceval S prosthesis in both positions (1.64±0.02 vs. 1.62±0.02 cm2, p=n.s.) (Figure 2A, Figure 4B). This was also seen for both positions of the MCVE (1.73±0.01 vs. 1.73±0.01 cm2, p=n.s.) (Figure 3A, Figure 4B). However, it is important to note that, when the MCVE was implanted into the Perceval S, the lower position was unstable and the valve dislocated to the higher position during perfusion experiments (Figure 5A, Figure 5B). The amount of total RV was not significantly different for the ESXT in both positions (3.59±0.81 vs. 4.0±1.02%, p=n.s.) (Figure 2A, Figure 4C). In addition, high-speed imaging of the lower implantation position showed impairment of ESXT leaflets by Perceval S leaflets (Figure 5E, Figure 5F).
HYDRODYNAMIC MEASUREMENTS FOR VIV IMPLANTATION IN THE 3f ENABLE RDV
When assessed within the 21 mm 3f Enable, the MPG over the ESXT was higher in the lower position compared with the higher position (15.62±0.12 vs. 12.68±0.17 mmHg, p<0.001) (Figure 2B, Figure 4D). Similarly, there was a difference in MPG for the MCVE favouring the higher position (13.18±0.3 vs. 11.58±0.28 mmHg, p<0.001) (Figure 3B, Figure 4D). The largest EOA was detected when the MCVE was implanted into the 3f Enable prosthesis in the higher position, which was significantly higher compared to the lower position (1.66±0.01 vs. 1.80±0.02 cm2, p<0.001) (Figure 3B, Figure 4E). Similarly, the higher position of the ESXT was superior to the lower position (1.57±0.02 vs. 1.72±0.01 cm2, p<0.001) (Figure 2B, Figure 4E). The amount of total RV was highest when deploying the ESXT in the lower position (7.96±2.77 vs. 3.99±0.95%, p=0.001) (Figure 2B, Figure 4F). Finally, high-speed imaging of the lower implantation position showed impairment of ESXT leaflets by 3f Enable leaflets (Figure 5G, Figure 5H, white arrows).
HYDRODYNAMIC MEASUREMENTS FOR VIV IMPLANTATION IN THE INTUITY RDV
The MPG within the 21 mm INTUITY prosthesis was higher for the 23 mm ESXT in the lower position compared with the higher position (16.54±0.33 vs. 11.45±0.13 mmHg, p<0.001) (Figure 2C, Figure 4G). Similarly, the MPG was slightly higher for the 23 mm MCVE in the lower position (14.92±0.29 vs. 13.79±0.28 mmHg, p<0.001) (Figure 3C, Figure 4G). Interestingly, the largest EOA was detected when the ESXT was implanted into the INTUITY prosthesis in the higher position, which was significantly higher compared with the lower position (1.61±0.01 vs. 1.82±0.01 cm2, p<0.001) (Figure 2C, Figure 4H) and both positions of the MCVE (Figure 3C, Figure 4H). Interestingly, high-speed imaging of the ESXT within the INTUITY revealed severe leaflet overhang by the INTUITY leaflets for the lower position (Figure 5I, Figure 5J). With the MCVE, the EOA was also higher in the higher position (1.56±0.01 vs. 1.64±0.01 cm2, p<0.001) (Figure 3C, Figure 4H). The amount of total regurgitation was highest when implanting the ESXT in the lower position (total regurgitation 13.4±1.2 vs. 6.3±1.4%, respectively; p<0.001) (Figure 2C, Figure 4I).
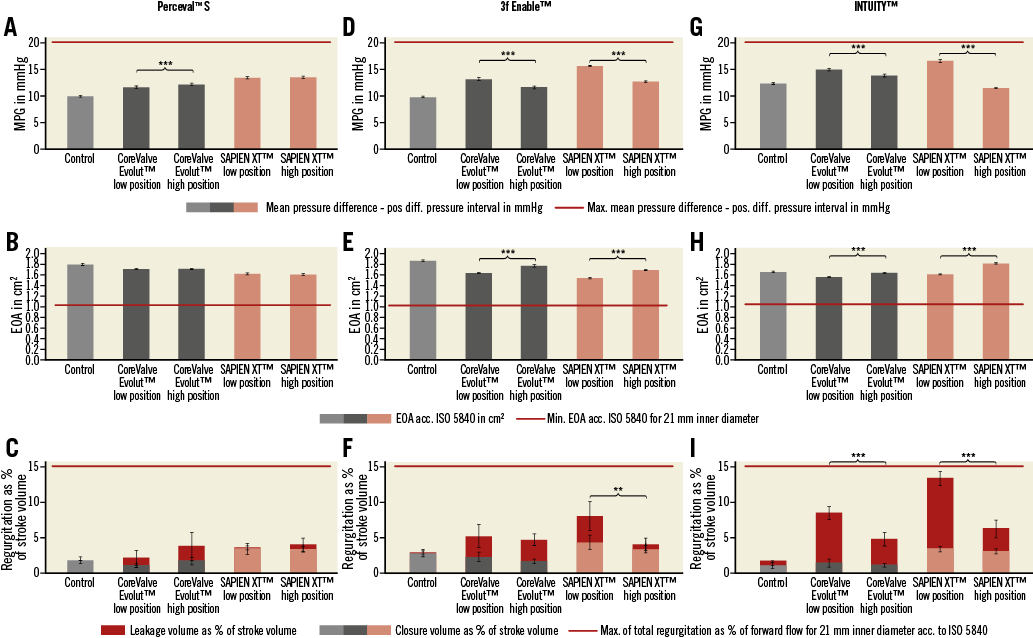
Figure 4. Functional performance of the THV implanted in the three RDV. Hydrodynamic measurements including MPG, EOA and regurgitation volume during perfusion conditions in accordance with ISO for the ESXT and MCVE implanted into the Perceval S (A-C), 3f Enable (D-F) and INTUITY (G-I). Baseline measurement of the native RDV alone (control) and the corresponding ViV combinations with the ESXT and MCVE are shown. Regurgitation volume is shown as percentage of stroke volume and comprises the closure volume and leakage volume. **p<0.01. ***p<0.001.
TECHNICAL CONSIDERATIONS
All ViV positions except one were technically feasible, as implantation of the MCVE into the Perceval S in the lower position was possible but the valve dislocated during the hydrodynamic test as deployment of the upper crown of the MCVE was impaired by the Perceval S stent (Figure 3A, Figure 5D), resulting in an upwards movement of the MCVE into the higher position (Figure 3A, Figure 5A, Figure 5B). In addition, during retrograde delivery of the MCVE, the upper stent segments of the Perceval S mechanically interfered with the delivery device, making accurate placement of the valve difficult (Figure 5C), whereas such difficulties were not observed in the higher position (Figure 5D).
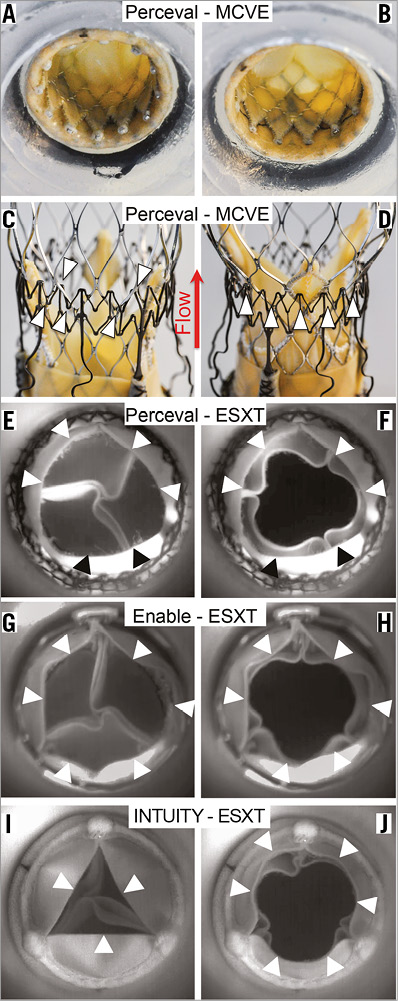
Figure 5. Technical considerations. Ex vivo photographs of a low implanted MCVE within the Perceval S (A) resulted in cranial migration of the MCVE during perfusion experiments (C). Entanglement of the MCVE during retrograde positioning within the Perceval S (B, D). High-speed analysis of leaflet kinetics during perfusion of ESXT in RDV revealed leaflet overhang and leaflet interactions causing incomplete opening of the RDV and THV leading to reduced EOA. Arrowheads indicate leaflet margins of Perceval S in diastole (E) and in systole (F), 3f Enable in diastole (G) and in systole (H), and INTUITY in diastole (I) and in systole (J). The red arrow indicates direction of flow.
Discussion
This is the first study to compare the in vitro hydrodynamic performance and leaflet kinetics of the two THVs currently approved for use in degenerated bioprostheses, the MCVE and the ESXT, within the two sutureless aortic heart valves commercially available in Europe, the Perceval S and the INTUITY, the 3f Enable having been recently withdrawn from the market by the company. Nevertheless, the performance data and functional results of this prosthesis remain included in this manuscript as already implanted 3f Enable prostheses may eventually degenerate and require a ViV procedure. The ISO 5840-3 requires an EOA of at least 1.05 cm², a total RV of less than 20% and a transvalvular leakage of less than 10% as a minimum requirement for a 21 mm THV18. Clinically, a post-procedural MPG below 20 mmHg is defined as device success according to the updated VARC-2 criteria19. Here we show that both THVs showed satisfactory hydrodynamic results after ViV implantation in all three types of RDV. However, considerable differences in ViV performance were observed depending on the individual design of the RDV as well as the technical feasibility of positioning the devices. The current study therefore provides detailed information for the transcatheter treatment of all three RDV, resulting in clinically valuable guidance on how to position the THVs within the RDV optimally.
The implantation of RDV (also known as sutureless valves) has emerged as an alternative to conventional valves in recent years20,21. The theoretical advantages of these valves include a safe and rapid implantation using modern deployment techniques, which is thought to facilitate minimally invasive approaches and also reduce cardiopulmonary bypass and aortic cross-clamp times13,21,22. Due to the biologic nature of these valves, a number of patients will eventually require a repeat intervention such as redo surgery or a transcatheter ViV procedure9,23. However, there is only very little clinical experience with treatment of RDV10,24-26. The “Valve-in-Valve App” contains recommendations for the selection of a THV for the RDV27. These are a unique help in the hands of an experienced physician, but crucial information regarding the expected haemodynamic performance or potential leaflet interactions between the “old” and “new” valves is not provided27.
The latter aspect is particularly important as the Perceval S, 3f Enable and INTUITY have very special and unique design features that potentially have an impact on the access route and valve positioning. The distal outflow ring of the Perceval S and the high stent profile of the 3f Enable represent potential mechanical obstacles for a retrograde approach. Furthermore, we observed that implantation of the MCVE into the Perceval S in the lower position resulted in dislocation of the MCVE during the hydrodynamic test, as deployment of the upper crown of the MCVE was impaired by the Perceval S stent, resulting in an upward migration of the MCVE to the higher position. Therefore, this position cannot be recommended for the clinical treatment of a dysfunctional Perceval S with the MCVE. As the 3f Enable stent is somewhat shorter, this phenomenon was not seen during MCVE implantation in the lower position. In contrast, the INTUITY has no upper stent frame exceeding the commissural struts and therefore presents no obvious obstacle for a retrograde delivery. Finally, in the initial learning phase, our team found it helpful to use a transapical approach for treatment of the Perceval S and 3f Enable, as this is technically easier, safer and facilitates a more precise positioning.
Interestingly, measurements within the three RDV showed fairly similar MPGs when comparing the MCVE and the ESXT in the higher position. Therefore, it seems that the advantage of the supra-annular valve function of the MCVE enabling a higher EOA than after implantation of the ESXT is not as pronounced as observed after ViV procedures into conventional surgical prostheses. Two explanations may account for this phenomenon. First, in particular the high stent design of the 3f Enable and Perceval S impaired full expansion of the upper stent part of the MCVE, even in the high implantation position (Figure 3A). Second, we have previously shown that the diameter of the nitinol ring of the Perceval S at the annular level was increased after implantation of the ESXT as a ViV procedure10. This observation may also be valid for the 3f Enable, as this valve also has a nitinol stent, therefore possibly allowing a further increase of the diameter after implantation of a THV.
Our findings suggest that the outcome after ViV treatment of an RDV may differ from those after treatment of conventional surgical bioprostheses, as reported in the VIVID registry9. According to the VIVID registry, one complication that occurs more often after a ViV procedure than after standard TAVI is elevated post-procedural gradients. The majority of the treated patients in the VIVID registry (almost 80%) presented with a stented surgical bioprosthesis. Therefore, the most obvious reason for this observation is the stiffness of the stent of the bioprosthesis which limits the degree of THV expansion within the bioprosthetic compartment, even after post-dilation. Therefore, the results of the VIVID registry cannot be transferred to RDV without caution, and further experience needs to be gained. As these new types of valve have different design features compared with conventional surgical valves, they merit further separate investigation.
Our preclinical in vitro analysis also revealed differences in valvular regurgitation among the three prostheses. Whereas the amount of RV was highest with the ESXT within the INTUITY, the RV was lowest for the ESXT when used within the Perceval S. Comparable RV was observed for the ESXT or MCVE when implanted in the 3f Enable or Perceval S, which have a similar design. The Perceval S and 3f Enable, which have native bovine or equine heart valves, seem to provide a good seal, while the INTUITY’s leaflets made of bovine pericardium are stiffer, resulting in a higher paravalvular leakage. However, the RV was relatively low in all tested combinations, and clinically regurgitation is not a predominant issue in ViV procedures.
The VIVID registry revealed an increased risk of coronary obstruction only for some heart valves, in particular for tissue valves with externally mounted leaflets9. In these circumstances, the higher risk of coronary obstruction is most likely due to the short distance between the valve leaflets that are pushed aside and the coronary ostia. In this regard, the three investigated RDV have distinct differences and need to be analysed individually: the top half of the INTUITY is constructed in a very similar way to the Edwards PERIMOUNT (Edwards Lifesciences Inc.) in that the leaflets are attached to the inside of the stent. Therefore, even in a high position of the THV, the risk of coronary obstruction is low. Similarly, the leaflets of the 3f Enable are attached to the inside of the stent frame. However, the stent design is long and the leaflets are also fairly large, but the supra-annular nitinol struts act like a spacer, keeping the risk of coronary obstruction low. The Perceval S is equipped with three sinusoidal struts that protrude into the sinus of Valsalva, therefore creating space between the leaflets and the coronary ostia, something which is also protective of coronary obstruction.
The newest-generation balloon-expandable SAPIEN 3 (ES3) has now widely replaced the ESXT in clinical practice. We think that the ES3 is as suitable for RDV ViV procedures as the ESXT. The main recommendation based on our in vitro experiments is that the higher implantation position will yield superior haemodynamic results, especially for balloon-expandable valves with a short stent design. This high implantation position is even more critical for the ES3, as the major design feature difference compared to the ESXT is the additional outer skirt to prevent paravalvular leakage (PVL). However, in a ViV setting, PVL is not the predominant issue, as the frame of the surgical valve usually provides an excellent sealing, except for certain surgical valves such as the Trifecta™ (St. Jude Medical, St. Paul, MN, USA) and Mitroflow (Sorin [now LivaNova]). In this context, the external ES3 sealing skirt adds additional “material” to the inner annular orifice area, resulting in a possible impairment of haemodynamics.
Limitations
This study has several limitations attributed to its design. First, new and non-degenerated RDV were assessed, whereas in the clinical context ViV TAVI is mostly performed in bioprostheses which have undergone valve degeneration demonstrating stenosis or regurgitation. In particular, calcified native leaflets and pannus may alter haemodynamic results that may have an effect on patients’ outcome. Second, assessment of hydrodynamic measurements was carried out under flow conditions simulating physiological cardiac output (5 L/min) using standard reference parameters according to ISO regulations. Therefore, they may not resemble the entire complexity of actual in vivo conditions. However, we were able to show in the past that the parameters obtained with this model correlated very well with the actual patients’ haemodynamics10.
Conclusions
In conclusion, we have tested the suitability and functional performance of the MCVE and ESXT for ViV procedures in all currently available RDV, including the recently discontinued 3f Enable. Here we show that hydrodynamic results and leaflet kinetics were better for both THVs when implanted in the higher position and in line with ISO standards. Furthermore, our data suggest that a very precise positioning of the THV within the RDV is more crucial for the ESXT in order to achieve an optimal functional result.
Finally, preclinical ViV simulations might help to understand the mechanisms affecting the haemodynamic performance of a particular ViV configuration and therefore enable the clinician to decide on the most suitable valve combination and the correct implantation heights to avoid risks and maximise patient safety.
| Impact on daily practice In the future, at least a certain proportion of patients treated with RDV can be expected to require a ViV procedure due to RDV failure and increased risk for redo surgery. However, experience with ViV treatment of RDV is scarce. Here, we present X-ray images of ViV positions that are safe and feasible, and that promise the best functional result in terms of THV performance after implantation into small RDV. |
Funding
The study was supported by the ADUMED-Stiftung.
Conflict of interest statement
B. Fujita has received travel compensation from Edwards Lifesciences and Symetis. S. Scholtz is a proctor for Direct Flow Medical, has received speaker honoraria from Medtronic, and has received travel compensation from Edwards Lifesciences, Medtronic and Direct Flow Medical. J. Börgermann is a proctor and consultant for Medtronic, has received speaker honoraria from Symetis, and has received travel compensation from Medtronic and Symetis. U. Steinseifer is a consultant for JenaValve. S. Ensminger is a proctor and consultant for Edwards Lifesciences, a proctor and member of the SAB of JenaValve, and has received speaker honoraria from Edwards Lifesciences and Symetis, and travel compensation from Edwards Lifesciences. The other authors have no conflicts of interest to declare.

