Abstract
Aims: The aim of this study was to evaluate transcatheter aortic valve-in-valve (ViV) implantation performance in rapid deployment (ViVr) vs. conventional (ViVc) surgical heart valves.
Methods and results: A multicentre registry was developed as part of the VIVID international registry. A total of 30 ViVr patients (Perceval, n=24, ATS 3f Enable™, n=5, and the INTUITY, n=1) were evaluated and compared with 2,288 ViVc patients. Propensity score (PS) matching was performed to adjust further for bias. Compared with ViVc, ViVr patients presented twice as early after surgical heart valve (SHV) implantation (55.2±36.1 vs. 118.4±57.7 months, p<0.001), were more commonly female (82.8% vs. 41.3%, p<0.001), and had shorter body stature and reduced body weight (p<0.05 for both) prior to PS. Implantation was successful in all ViVr cases and, compared with ViVc, was associated with equally favourable haemodynamic outcomes (mean gradient: 14.6±8.3 vs. 16.2±8.9 mmHg, p=0.356; regurgitation ≥mild: 3.7% vs. 5.2%, p=0.793). Periprocedural complication rates were similar and low in both groups. There was no coronary obstruction event in any ViVr case; one patient (3.6%) died during one year of follow-up.
Conclusions: ViVr appears effective, safe and associated with favourable haemodynamic outcome.
Abbreviations
| SHV | surgical heart valve(s) |
| SHVc | conventional surgical heart valve |
| SHVr | rapid deployment surgical heart valve |
| ViV | valve-in-valve implantation |
Introduction
Bioprosthetic surgical heart valves (SHV) are less thrombogenic than mechanical SHV and do not require lifelong anticoagulation therapy. Nevertheless, bioprostheses eventually tend to degenerate and to require subsequent treatment1,2,3,4. Although still the benchmark, valve reoperation carries substantial risks5,6,7,8,9,10. Therefore, transcatheter heart valve (THV) implantation into a degenerated bioprosthetic aortic SHV (valve-in-valve [ViV] procedure) has evolved as a viable, less invasive strategy in suitable cases of SHV failure. Rapid deployment SHV (SHVr) are a relatively novel subcategory of aortic bioprostheses. Similar to conventional SHV (SHVc) surgery, SHVr surgery requires native aortic valve excision and annular decalcification but it avoids the need for permanent sutures at the decalcified annulus. SHVr may be particularly beneficial in reducing surgical procedure time and are also easier to implant, with smaller non-sternotomy incisions. Moreover, rapid deployment valves (RDVs) are designed to allow larger residual effective orifice area in patients with small aortic annuli, as avoiding a sewing ring typically enables upsizing the implanted device by one or two sizes. Thus, the rationale for their use lies in their potential to facilitate minimally invasive surgical approaches, reduce operation time, and allow implantation in severely calcified and particularly narrowed aortic roots11. Consequently, SHVr are increasingly being used, mainly offered to older patients at higher surgical risk and in more complex surgeries12,13,14. The absence of sutures could, on the other hand, have potential disadvantages that include paravalvular leakages, valve dislocation, and stent infolding. All may cause valve failure and require reintervention15,16. Because of their potential benefits, SHVr prostheses are often implanted in patients already at relatively high risk, and therefore re-operating these patients is a high-risk procedure. Hence, ViV intervention may potentially play a major role in suitable patients, if and when SHVr failure should occur. Currently, ViV in degenerative SHVr (ViVr) experience is very minimal, with only a few cases reported in the literature15,16,17. The purpose of this study was to evaluate the ViVr procedure and outcomes and to compare them against ViV in conventional SHV (ViVc).
Methods
STUDY POPULATION AND DESIGN
Centres were solicited to participate through the Valve-in-Valve International Database (VIVID) registry as described in detail previously18. Briefly, the VIVID registry is a global data collection of ViV procedures in the aortic, mitral and tricuspid positions. Since 2010, the registry has been prospectively collecting data from centres in Europe, North America, South America, Africa, Oceania, and the Middle East using a dedicated case report form. Centres were requested to submit consecutive data for successful and unsuccessful procedures, as well as for patients who underwent catheterisation planned for ViV but in whom no implantation was attempted. The institutional review board or ethics committee at each participating centre approved the submission of data. In the following analysis, all cases of ViV in the aortic position were included, and ViVr patients were compared against ViVc patients.
STUDY MEASURES AND ENDPOINTS
Data collected included basic demographic, historical and diagnostic information, procedural details and follow-up information related to THV function, reintervention, adverse events and clinical status. Major clinical endpoints were assessed according to the Valve Academic Research Consortium-2 consensus document19. Post-implantation haemodynamic data were obtained from either intraprocedural or first post-procedural echocardiography, with additional echocardiography performed during follow-up. The primary outcome was defined as 30-day mortality. Secondary outcomes were defined as stroke, need for a second valve, >mild residual aortic regurgitation and residual mean aortic valve gradient (mmHg) higher than 20 at 30 days, as well as mortality at one year. Other VARC-2-defined 30-day outcomes were also measured and reported.
STATISTICAL ANALYSIS
Categorical variables are reported as n (%) and continuous variables as mean (±standard deviation) or median (interquartile range [IQR]), depending on variable distribution. Group comparisons were analysed using the Student’s t-test or Wilcoxon rank-sum test. The χ2 and Fisher’s exact tests were used to compare categorical variables. The time-to-event curve was calculated using the Kaplan-Meier method. The Student’s t-test was used to compare normally distributed continuous variables and the Wilcoxon rank-sum test was used for variables not normally distributed. The χ² and Fisher’s exact tests were used to compare categorical variables. To account for missing data, we used multiple imputation, and pooled the results across imputations. A two-sided p<0.05 was considered statistically significant. A propensity score matching based on patients’ calculated propensity (0.0-1.0) of having had a ViVr procedure was also conducted. The propensity score was estimated using a “saturated” logistic regression adjusting for preprocedural factors including five continuous terms (age, body surface area [Mosteller formula], body mass index, left ventricular ejection fraction, and mean aortic valve gradient), four binary or categorical terms (patient gender, mechanism of failure, whether the patient had renal failure, and whether the patient had diabetes), all two-way interactions of the covariates (continu-ous-continuous, continuous-categorical, and categorical-categorical), and all squared and cubed terms of the continuous variables. Nearest neighbour propensity score matching with replacement was performed using the teffects psmatch command in Stata 14.2 (StataCorp, College Station, TX, USA).
Results
Between April 2007 and January 2018, there were 30 ViVr implantations performed at 17 out of 135 sites participating in the VIVID registry, using: 24 Perceval (Sorin Biomedica, Saluggia, Italy), five ATS 3f Enable (Medtronic, Minneapolis, MN, USA) and one INTUITY (Edwards Lifesciences, Irvine, CA, USA) valves. No ViVr was performed between 2007 and 2011, nine were performed between 2012 and 2014, and 18 were performed between 2015 and 2017. During these years, 2,288 ViVc implantations were performed at the 135 centres participating, and these were included for comparative purposes.
BASELINE CHARACTERISTICS AND CLINICAL PRESENTATION
The mean age of ViVr patients was 80.1±7.6 years and their mean STS score was 9.5±7.0%. Compared with the ViVc group of patients, ViVr patients were more commonly female (82.8% vs. 41.3%, p<0.001) with shorter body stature (159.9±7 vs. 167.5±9 cm, p<0.001) and reduced body weight (69.0±13 vs. 75.9±17.2 kg, p=0.035). The surgical risk as assessed by the EuroSCORE II and STS score was similar between the groups (14.7±6.6% vs. 14.3±9.4%, p=0.660 and 9.5±7.0% vs. 8.8±8.1%, p=0.855, for ViVr and ViVc, respectively), as were other baseline characteristics, all presented in Table 1.
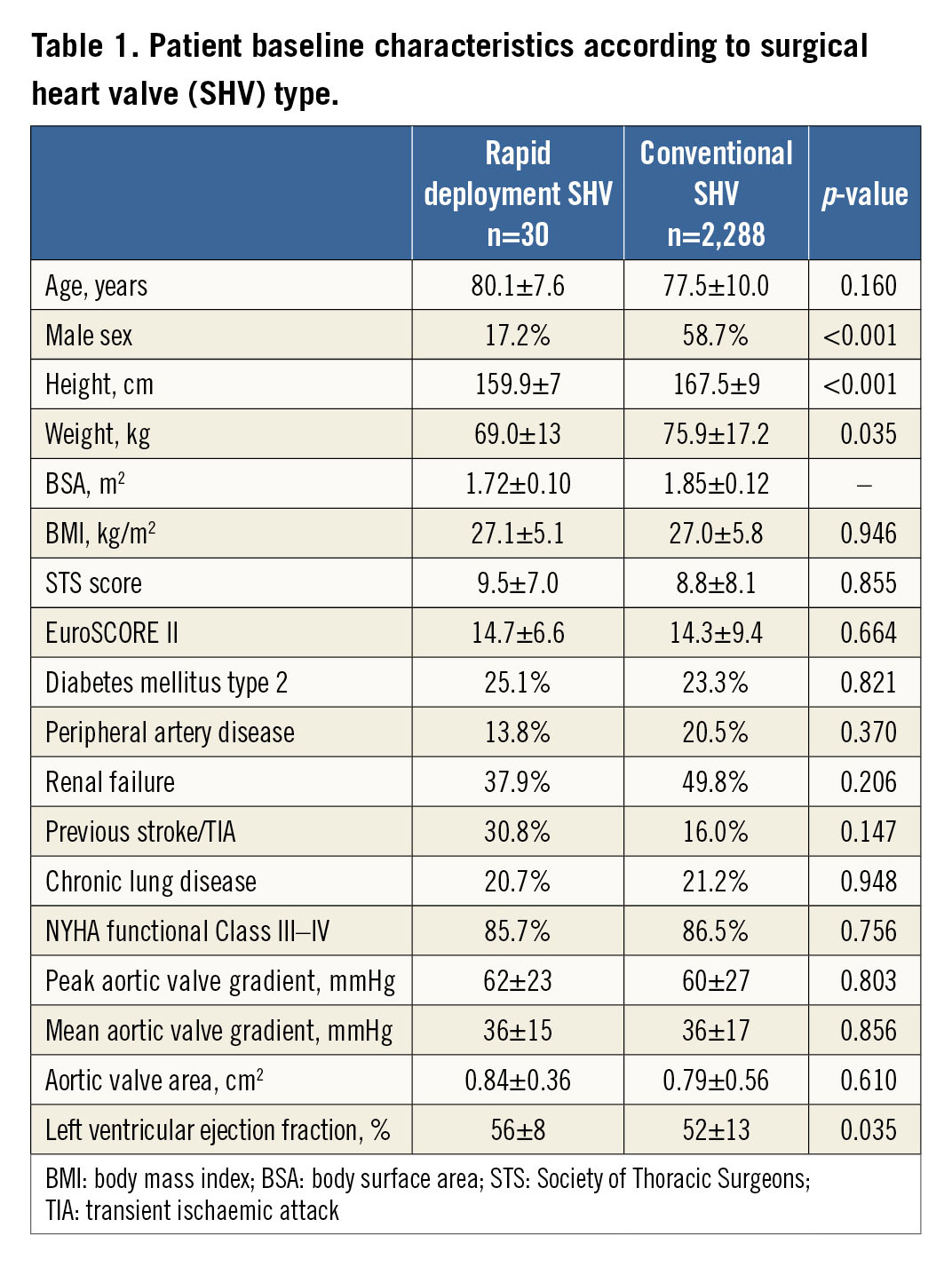
Compared with ViVc patients, ViVr patients presented more commonly with a combined (stenosis and regurgitation) mechanism of SHV failure (60% vs. 31%, p=0.003) and at shorter surgical AVR to ViV time interval (55.2±36.1 vs. 118.4±57.7 months, p<0.001). The SHV label size (23.0±1.5 vs. 23.3±2.2 mm, p=0.338), baseline aortic valve area (0.84±0.36 vs. 0.79±0.56 cm2, p=0.610) and mean transvalvular gradients (36±15 vs. 36±17 mmHg, p=0.856) were similar between ViVr and ViVc patients, respectively (Supplementary Table 1).
TECHNICAL PROCEDURAL CHARACTERISTICS AND HAEMODYNAMIC OUTCOME
Figure 1 shows each SHVr type along with ViV procedural illustrations. In both groups, the majority of procedures were performed using the transfemoral access (72.4% ViVc vs. 77.8% ViVr, p=0.579). Compared with ViVc, ViVr procedures were more frequently carried out utilising a balloon-expandable THV (70% vs. 42.2%, p=0.022), with a trend towards using a smaller THV diameter size (23.9±2.1 vs. 24.6±2.2 mm, p=0.078).
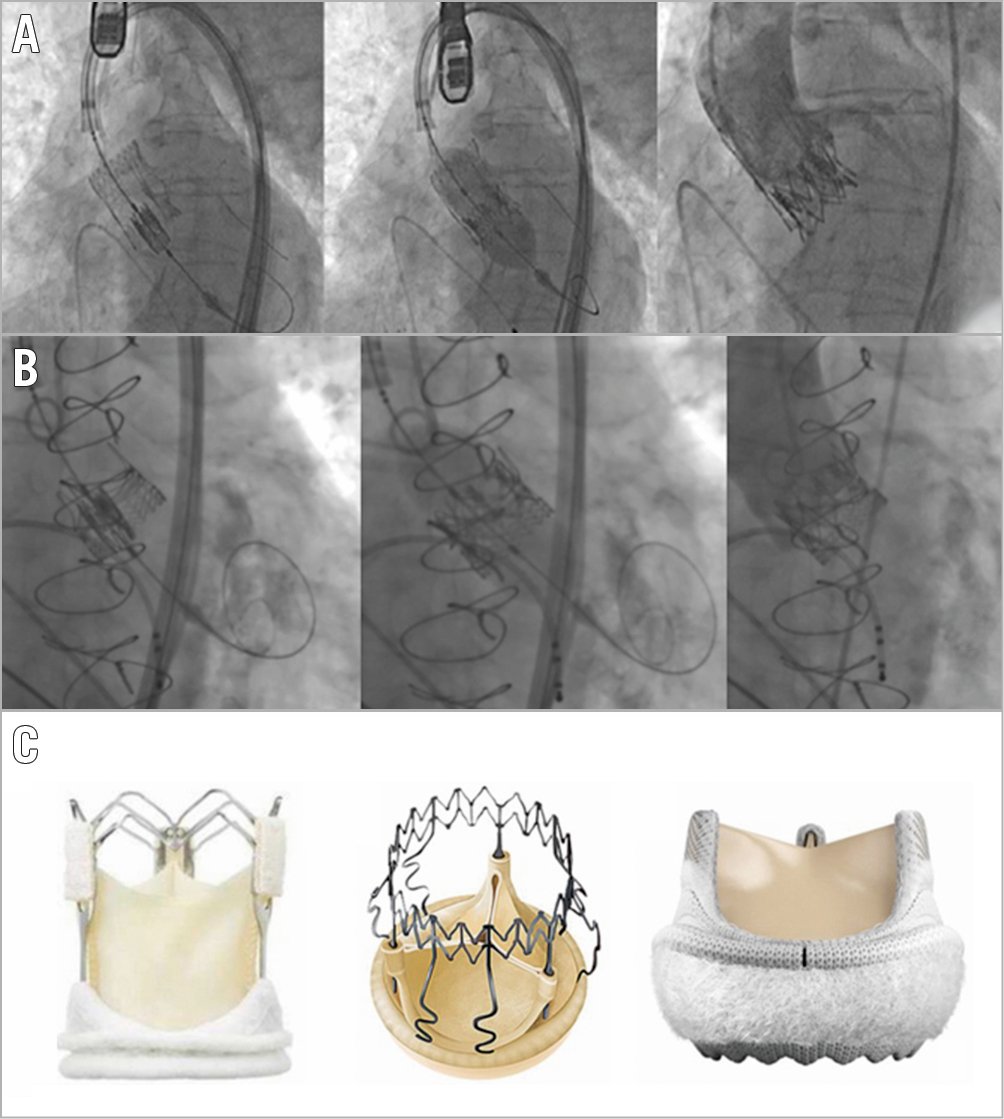
Figure 1. Transcatheter aortic valve implantation into (A) Perceval and (B) INTUITY sutureless surgical heart valves. (C) Left: ATS 3f Enable (Medtronic); middle: Perceval (Sorin Biomedica); right: INTUITY (Edwards Lifesciences).
Implantation was successful in all ViVr patients. Compared with ViVc, aortic valve area after ViVr was numerically, but not statistically, larger (1.28±0.69 vs. 1.02±0.78 cm2, p=0.124) and accompanied by lower maximal (21.2±10.7 vs. 28.9±14.8 mmHg, p=0.013) and similar mean (14.6±8.3 vs. 16.2±8.9 mmHg, p=0.356) residual transvalvular gradients. Residual aortic regurgitation was ≥mild in one (3.7%) ViVr and in 107 (5.2%) ViVc patients, p=0.793.
EFFICACY AND SAFETY OUTCOMES
VARC-2-defined periprocedural complication rates (i.e., stroke, kidney injury, vascular complications, etc.) were similar in patients who underwent ViVr vs. ViVc, and low in both groups (Table 2). In the ViVr patient group, at 30 days there was one (3.6%) case of a second THV implantation, three (11%) cases of permanent pacemaker implantation, and no case of coronary artery obstruction. One patient (3.6%) died during 30-day follow-up, and there were no additional deaths during one-year follow-up. The Kaplan-Meier survival curve in respect of SHV type is presented in Figure 2.
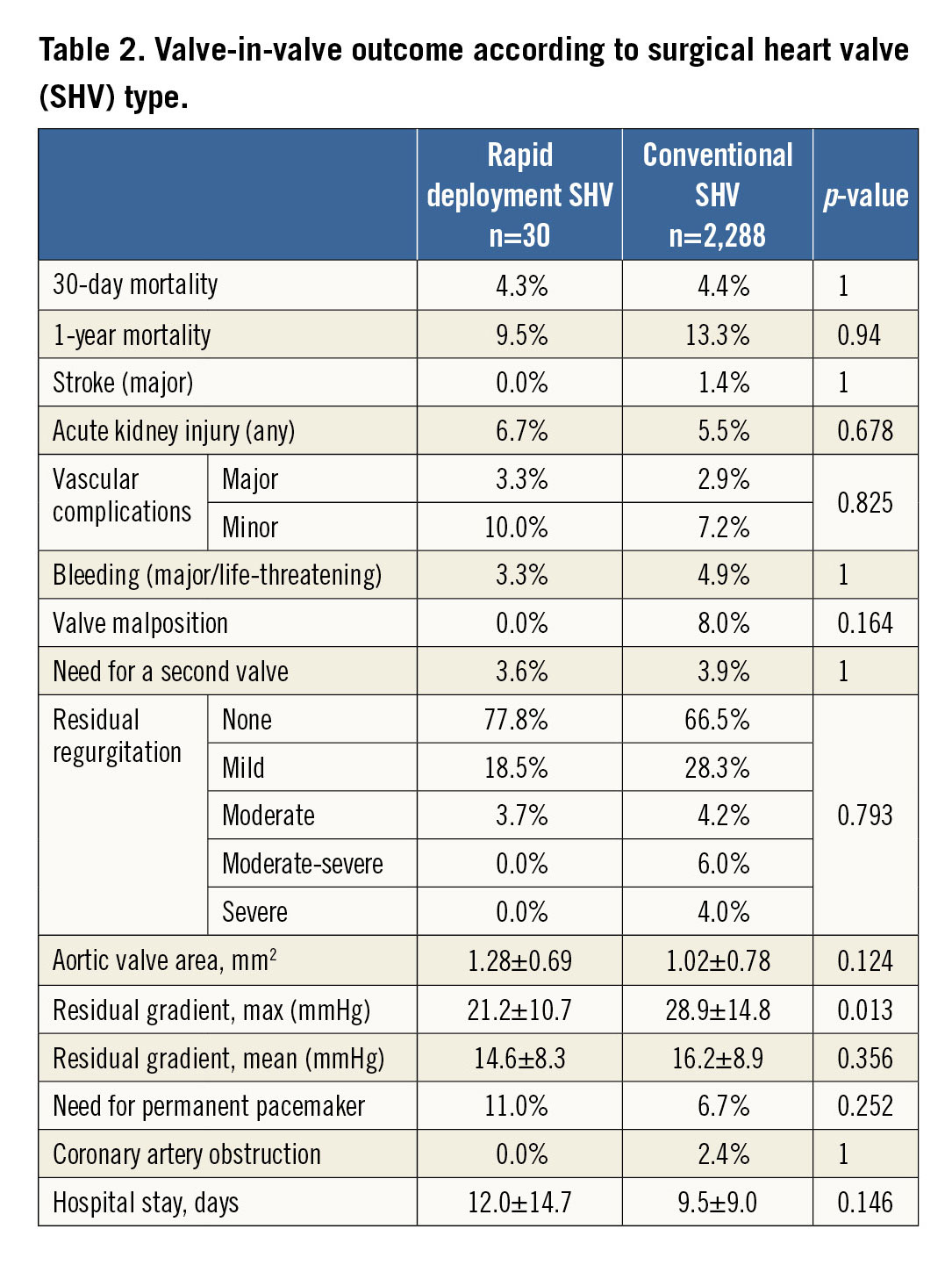
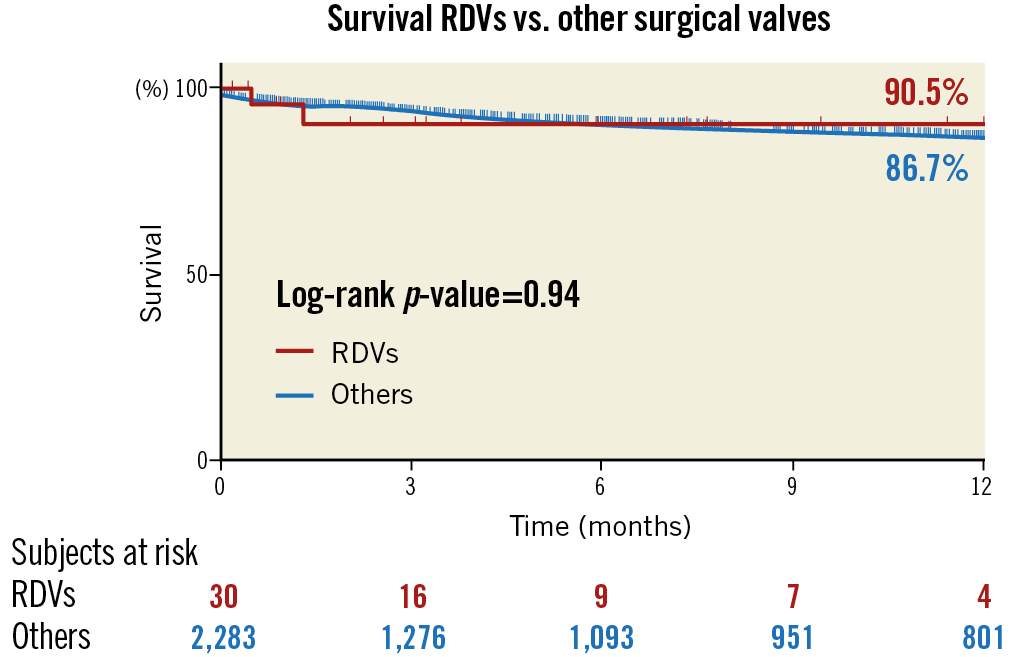
Figure 2. Kaplan-Meier survival curve after transcatheter aortic valve implantation into a failed surgical heart valve bioprosthesis (valve-in-valve) in respect of SHV type. RDVs: rapid deployment valves
Symptomatic improvement (vs. baseline) assessed by NYHA functional class de-escalation was evidenced in 95.5% of the patients at 30 days (p<0.001) and remained steady at one year, with no difference in ViVr vs. ViVc patients.
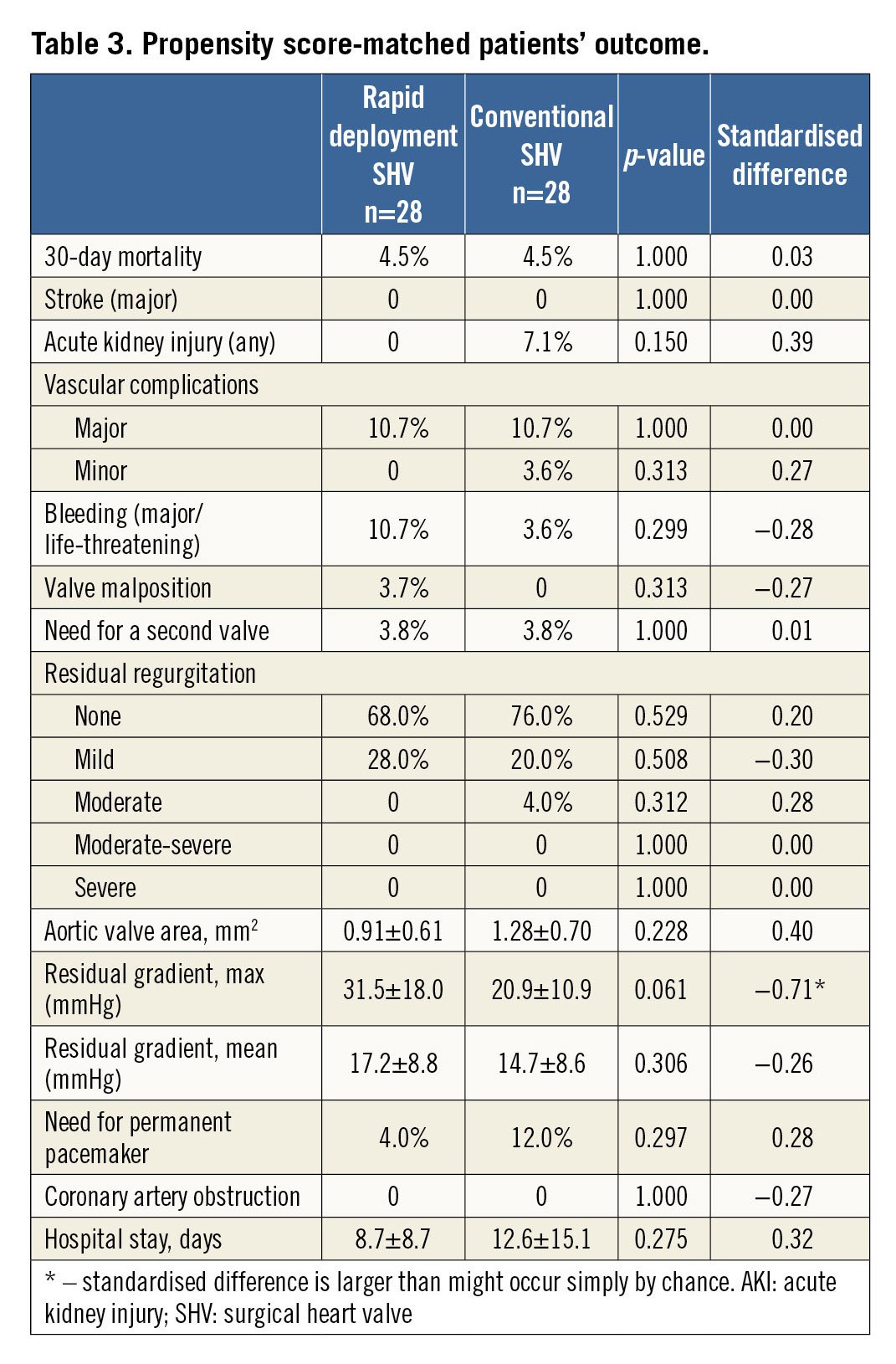
Using propensity score matching across imputation, 56 patients (28 pairs) were found eligible for 1:1 ViVr to ViVc matching. Baseline covariates and procedural characteristics were evenly distributed across the groups, including patients’ sex, body stature and body weight, yet excluding surgical AVR to ViV time interval (65.3±30.9 months ViVr vs. 126.4±69.9 months ViVc, p=0.001) (Supplementary Table 2). Thirty days after the procedure, aortic valve area was 1.28±0.70 vs. 0.91±0.61 cm2 (p=0.228), maximal residual transvalvular gradient was 20.9±10.9 vs. 31.5±18.0 mmHg (p=0.061) and mean residual gradient was 14.7±8.6 vs. 17.2±8.8 mmHg (p=0.306) in ViVr vs. ViVc, respectively. No significant difference was observed between the groups in terms of clinical outcomes, including 30-day mortality, cerebrovascular events, vascular complications, acute kidney injury or need for permanent pacemaker (Table 3).
Discussion
The first ViV inside SHVr cases were only recently described and the overall ViVr experience is so far limited15,16,17. Incidence of SHVr deterioration at one year is reported to be 0.4%14, yet no strict follow-up criteria have been applied and there are no data yet regarding the long-term deterioration rate. If we expect SHVr to have durability/longevity comparable to SHVc, then we are likely to witness an increase in the incidence of patients presenting with SHVr failure over the next few years. This assumption motivated us to look into the largest global ViV registry so far and to solicit ViVr cases, in order to learn about this important patient cohort.
Our report suggests that ViVr is feasible and safe in selected cases of degenerated SHVr, with results similar to those seen in conventional ViV patients. In this analysis, several observations are important and deserve additional consideration.
As SHVr are frequently utilised to augment surgical AVR in patients with a small left ventricular outflow tract, it is not surprising that overall ViVr patients were more commonly female and had shorter body stature and reduced body weight. Albeit that they were 2.5 years older (not statistically significant), ViVr patients’ baseline characteristics were similar to ViVc characteristics excluding the above aspects. However, the clinical presentation differed significantly between the two groups, with twice as short a time lapse between the surgical AVR and the ViV procedure in patients with SHVr compared to SHVc, and with SHVr patients presenting more often with SHV failure due to regurgitation compared to SHVc, which more commonly presented with predominant stenosis. Even after PS-matched analysis ensuring evenly distributed baseline parameters across the two groups, surgical AVR to ViV time remained shorter in SHVr patients. Although our study is unable to identify factors explaining the degeneration process systematically, this point is mandatory and hypothesis-generating, as traditional SHVc function usually deteriorates due to leaflet failure secondary to leaflet calcification, pannus, tears, etc., while SHVr may be more susceptible to frame failure and develop paravalvular leak due to partial collapse of the non-sutured valve frame15,16, which may also lead to valvular stenosis and/or regurgitation due to leaflet deformity, as demonstrated in Figure 3.

Figure 3. Bioprosthetic degeneration of a Perceval sutureless valve in one of the patients in the study. A) Computed tomography demonstrating stent disfigurement with the valve in-folding at the right and non-coronary sinuses. B) Echocardiography demonstrating severe valve regurgitation (left: colour Doppler showing a mosaic pattern of diastolic flow; right: continuous wave Doppler showing transvalvular gradients and the regurgitation flows). C) After valve-in-valve: no residual leakage (left) and normal pressure gradients (right).
ViVr patients showed a numerically more favourable residual valve area and lower residual gradients. These haemodynamic results were obtained even though SHV area and transvalvular gradients were similar between the groups at baseline, the THV size utilised was similar (with a trend for being smaller in ViVr), and despite the fact that ViVr used a higher proportion of balloon-expandable to self-expanding THVs (self-expanding THVs are associated with a more supra-annular position, in which the frame is less constrained by the SHV ring/frame, thereby enabling a larger residual effective orifice area and reduced residual gradients after ViV implantation)20. These favourable ViVr haemodynamic results were also maintained after propensity analysis, hence are less likely to be related to group dissimilarities in sex, body stature, or weight. Alternatively, several other explanations may be more applicable. To begin with, the failure mechanism of SHVr less commonly involved pure stenosis, which is associated with lower residual valve area and higher residual gradients compared with SHV regurgitation or stenosis-regurgitation combination13. Additionally, differences between SHV structure and mechanics may hypothetically contribute to more advantageous haemodynamics after ViVr compared to ViVc. As opposed to SHVc, SHVr frames lack the restrictive sewing ring (a recent target for fracturing by some operators)21 and therefore may be more elastic and extendable by the THV, allowing the ViV to restore a more favourable frame geometry. In this regard, ViVr is similar to THV in stentless SHV and THV-in-THV implantation (redo transcatheter aortic valve replacement), which are also associated with favourable haemodynamic outcomes22.
The clinical outcome of ViVc is generally good, and comparison with benchmark transcatheter native valve aortic valve replacement (AVR) shows ViV to be both safe and effective overall23. Although the number of ViVr cases in the current study is still too small, this analysis supports the safety and efficacy of this procedure, as it demonstrates ViVr to be associated with outcomes comparable to ViVc, a low mortality rate, low complication rate and symptomatic benefit. It is worth pointing out that there were no coronary artery occlusion events within this series of ViVr patients. Interestingly, the Perceval (which constituted 80% of ViVr cases) and the Freedom Solo™ SHV (Sorin Biomedica) actually use the same leaflets, which are very long and thought potentially to jeopardise the coronary ostia and thus at least in part be responsible for the high coronary obstruction risk associated with ViV within the last24. We do not yet know if the zero rate of coronary obstruction events in our series is a consequence of patient selection (i.e., low coronary obstruction risk anatomy with wide aortic root sinuses, high coronary ostia, etc.) or whether the frame design of these devices somehow protects from this devastating complication.
Limitations
Our findings should be interpreted with caution and should be considered as hypothesis-generating, since our registry has inherent selection bias as it has collected a miscellany of patients chosen for ViV by the local teams of experts. Also, results can be subject to differences in mode of practice and documentation patterns at participating institutions. Importantly, the ATS 3f Enable and the INTUITY were under-represented, the overall number of ViVr patients is still low despite being the largest so far, and imbalance in size and baseline characteristics exists between the study groups. We tried to overcome these differences by supplementary analysis of propensity-matched cohorts, but other confounders may still have influenced the results.
Conclusions
ViV in selected patients with degenerated rapid deployment SHV is feasible, safe and associated with clinical and haemodynamic outcomes which are comparable to ViV in conventional SHV. Further evaluation is needed in larger cohorts of patients and with longer follow-up.
|
Impact on daily practice Transcatheter valve-in-valve implantation (ViV) is used to treat failed surgical aortic valve bioprostheses but was only anecdotally described in rapid deployment surgical valves (SHVr), which are increasingly being used. Compared to conventional ViV (ViVc), ViVr patients presented twice as early after surgery, and more commonly with aortic regurgitation. Implantation was successful in all ViVr cases and associated with favourable haemodynamic outcome, low periprocedural complications (including coronary obstruction) and a low one-year mortality rate. Although further evaluation with longer follow-up and larger cohorts is needed to assess ViVr fully, ViV might be a valid treatment option in selected patients with failed SHVr. |
Acknowledgements
The authors wish to acknowledge Alex Woersching, MS, for his statistical analysis contribution in this study.
Conflict of interest statement
M. Andreas reports being a proctor for Edwards Lifesciences and receiving scientific grants from Edwards, Abbott and Medtronic. J. Webb is a consultant for Edwards Lifesciences. R. Kornowski reports institutional research grants from Medtronic. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Table 1. Valve-in-valve procedural characteristics according to surgical heart valve (SHV) type.
Supplementary Table 2. Propensity score-matched patients’ baseline characteristics.

