Abstract
Aims: Reoperation for bioprosthetic heart valve failure is associated with significant morbidity and mortality, particularly in high-risk patients. Transcatheter valve-in-valve (VIV) implantation may offer a less invasive alternative. The aim of this study was to report our initial experience with transcatheter VIV implantation to treat degenerated tissue valves.
Methods and results: VIV implantation with the Edwards SAPIEN transcatheter heart valve (THV; Edwards Lifesciences Inc, Irvine, CA, USA) was performed in 18 high-risk patients (STS 8.2±5.2%; logistic EuroSCORE 37.4±20.8%) with symptomatic bioprosthetic failure (17 aortic, one mitral). Valve Academic Research Consortium (VARC) definitions were applied for endpoint adjudication. Transfemoral access was the preferred vascular approach (16 patients, with the mitral VIV delivered anterogradely through the femoral vein; one transaxillary and one transapical). The majority (83%) of procedures were performed under local anaesthesia and sedation. Device success was achieved in all but one patient who had a final transaortic gradient ≥20mmHg. Acute kidney injury occurred in three patients (Stage 3 in 1), life-threatening or major bleeding in four patients, while major vascular complications occurred in one patient. Permanent pacemaker implantation was required in two patients. There were no deaths or neurological events at 30-day follow-up. At a median follow-up of 11 months (interquartile range 6-16), the mortality rate was 5.6% and all patients were in NYHA class II or lower.
Conclusions: Transcatheter implantation of the Edwards THV within a degenerated aortic bioprosthesis, performed predominantly via the transfemoral route, is feasible and associated with good periprocedural and clinical outcomes in high-risk surgical patients.
Abbreviations
THV: transcatheter heart valve
VIV: valve-in-valve
VARC: Valve Academic Research Consortium
AKI: acute kidney injury
EuroSCORE: European System for Cardiac Operative Risk Evaluation
Introduction
Bioprosthetic heart valves are increasingly being used in preference to a mechanical alternative in younger adult patients, and have been associated with comparable long-term survival.1-6 The major advantage of tissue valves is the avoidance of the need for long-term anticoagulation with its inherent risks, particularly with regard to bleeding.1,2,7 However, the trade-off is an increased risk of late reoperation due to structural degeneration, which progressively increases with time.1,3,4,7,8 It is inevitable that, as more younger patients are successfully treated with tissue valves, and their life expectancy continues to increase, we will be faced with a growing number of degenerated bioprostheses requiring reoperation.9,10 However, redo valvular surgery is considered high-risk, a risk which is escalated further by older age and the concomitant comorbidity often associated with these patients, such as left ventricular dysfunction, renal insufficiency, pulmonary hypertension and the need for a concurrent cardiac procedure.11-13
Transcatheter heart valve (THV) implantation with the valve-in-valve (VIV) technique is emerging as a feasible, reproducible and low risk treatment option in high-risk or inoperable patients with degenerated tissue valves.6,9,10,14-18 Although not primarily designed for this application, percutaneous VIV implantation offers a less invasive alternative to conventional redo surgery, by avoiding redo sternotomy, cardiotomy, valve excision, and cardiopulmonary bypass. Furthermore, the existing bioprosthesis can be helpful in both the sizing and positioning of a THV prosthesis, by providing a well demarcated and often radiopaque, circular landing zone of known diameter (Figure 1). Clinical experience with the VIV technique is still quite limited, with only a few cases described worldwide using both the Edwards SAPIEN THV (Edwards Lifesciences Inc, Irvine, CA, USA)9,10,14,19,20 and the Medtronic CoreValve Revalving System (Medtronic Inc., Minneapolis, MN, USA).15-18 Webb et al have demonstrated the feasibility of THV implantation with the Edwards prosthesis in the aortic, pulmonary, mitral and tricuspid positions.10,19 However, the majority of published data using the Edwards prosthesis relates to VIV implantation via the transapical route.6,9,10,20 Indeed, many experts have a preference for the transapical approach as direct access to the valve may allow better control of the device during implantation. Thus, the objective of this study is to report our experience with the Edwards prosthesis for VIV implantation, in a centre where transfemoral placement is the preferred approach.
Methods
We evaluated 18 consecutive high-risk patients with symptomatic bioprosthetic valve failure who underwent a transcatheter VIV procedure in our institution. All patients were evaluated by a multidisciplinary heart team as having a very high operative risk or ineligible for redo valve surgery because of unacceptably high surgical risk scores, severe comorbidity, or patient frailty. The only exception was a young patient who opted for a transcatheter approach as a means of postponing the inevitable requirement for a mechanical prosthesis to such a time when the implications of anticoagulation would have less impact on his lifestyle. Bioprosthetic valve failure was defined as a valve area <1 cm2 or mean transaortic gradient >40 mmHg or mean transmitral gradient >10 mmHg and/or severe (grade 3–4+) prosthetic valve regurgitation as assessed by transthoracic and/or transoesophageal echocardiography.21 All patients provided informed consent for the procedure and subsequent data collection and analysis for research purposes.
Procedures and devices
All patients underwent thorough multimodality imaging including echocardiography and multislice computed tomography (MSCT) to evaluate: 1) the severity and mode of bioprosthetic failure, 2) the peripheral vasculature in order to determine the implantation approach, 3) the aortic root anatomy and bioprosthesis dimensions to facilitate sizing, positioning and strategic planning of the THV implantation, and 4) the coronary vessels or bypass grafts for significant coronary artery disease.22
Based on the prosthesis type and labelled valve size, we obtained the following information directly from the manufacturer: prosthesis type and anatomic characteristics, i.e., stented vs. stentless, type of base ring (flat or saddle-shaped), position and angulation of stent posts), internal (inner stent) diameter, and prosthesis height. The radiopaque components of the valve such as stent posts and sewing ring (landing zone and target for VIV implantation) were confirmed on fluoroscopy. On MSCT, we confirmed the measurements of the surgical bioprostheses and annular dimensions (in the case of stentless valves without radiopaque components) in order to exclude major discrepancies in valve size to that reported in the patient’s medical file. Finally, we verified on the pre-procedural imaging that there were no surgical stent posts, bulky calcifications or pannus overlying the coronary ostia. We also discussed all these specific issues with a cardiac surgeon who has significant experience in valve replacement. The physical characteristics and anatomy of surgical bioprostheses, their relevance to transcatheter VIV procedures and the diagnostic work-up for these procedures has been recently described extensively by Piazza et al.5
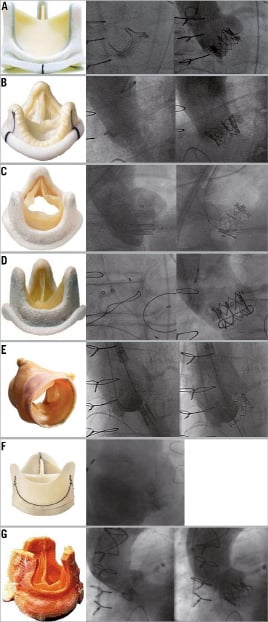
Figure 1. Case examples of the failed bioprosthetic valves treated by valve-in-valve implantation. Photo of the bioprosthetic aortic valve (left panel), fluoroscopic images of before (centre panel) and after (right panel) valve-in-valve implantation for the following failed bioprostheses: A) Carpentier-Edwards (patient 8); B) St. Jude (patient 7); C) Mosaic (patient 5); D) Hancock (patient 13); E) Freestyle (patient 4); F) Pericarbon (patient 10); G) Bravo (patient 11)
The transfemoral approach was considered the default strategy; however, if the peripheral vascular anatomy was unsuitable, the transaxillary or transapical approach was utilised. Procedures were performed in a catheterisation laboratory, with the exception of the transapical procedure, which was performed in an operating room. Depending on the preference of the physician, clinical conditions, and evaluation of the patient by the anaesthesiologist, the procedure was performed under either general anaesthesia or local anaesthesia with conscious sedation. However, local anaesthesia and conscious sedation was preferred with the transfemoral approach. The transapical procedure was carried out under general anaesthesia. The THV device was initially the Edwards SAPIEN until it was replaced by the Edwards SAPIEN XT in April 2010. THV size was based on the manufacturer’s specified internal dimensions of the failed bioprosthesis5,6 with a 23 mm and 26 mm Edwards SAPIEN valve for a bioprosthesis internal diameter measuring 17 to 23 mm and 24 to 26 mm, respectively. A VIV prosthesis was considered to be appropriately sized if its nominal external diameter matched or exceeded the internal diameter of the failed prosthesis. The mode of bioprosthetic failure (i.e., regurgitation vs. stenosis) needed to be taken into account when planning the procedure. Severe prosthetic aortic valve regurgitation can be associated with large stroke volumes and makes accurate VIV positioning difficult unless rapid pacing is performed during deployment.5 This is important to consider during the implantation of self-expanding THVs that are not routinely implanted with rapid pacing. However, the mode of bioprosthetic failure did not influence the choice of prosthesis or procedure as only Edwards SAPIEN valves were used in this series.
The technical aspects of THV implantation have been well described elsewhere by our group (Figure 2).22,23 However, there are certain procedural aspects that are specific to VIV procedures, which have been reported in the literature.9,10,16 Balloon valvuloplasty was not routinely performed before implantation even in stenotic valves. An attempt was made to cross the bioprosthesis with the Edwards predilatation balloon (20 mm for the 23 mm THV and 23 mm for the 26 mm THV) either uninflated or inflated at low pressure (2-3 atm). If the balloon easily crossed the bioprosthesis without resistance, THV implantation was performed without predilatation. For positioning, it was essential to find the angiographic view perpendicular to the bioprosthesis in order to minimise foreshortening. The prosthesis should be positioned slightly more towards the ventricle for aortic and atrium for the mitral valve with the THV overlapping the sewing ring of the surgical prosthesis, thus allowing more secure fixation.6,10,19 Valve implantation was always performing during rapid ventricular pacing at a rate sufficient to lower cardiac output and blood pressure, usually between 160-220 beats/min.
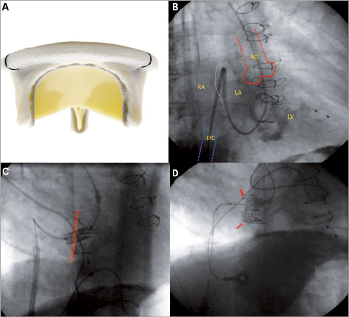
Figure 2. Transfemoral anterograde mitral valve-in-valve implantation. Modified from Reference 23. A: shows an example of the Carpentier-Edwards mitral bioprosthesis that degenerated in patient 15. B: after right femoral vein access and transseptal puncture, a Swan-Ganz catheter was manipulated across the mitral bioprosthesis and native aortic valve with the tip in the ascending aorta and then exchanged for a stiff wire. C: atrial septal dilatation was then performed; the NovaFlex delivery system was positioned in the right atrium, and the middle of the SAPIEN XT valve was positioned at the sewing ring without complete coaxiality. D: final result after valve-in-valve implantation with the SAPIEN XT valve overlapping the sewing ring of the surgical prosthesis.
The mitral VIV procedure was performed percutaneously via the transvenous-transseptal-anterograde approach.24 Briefly, after right femoral vein access, transseptal puncture was performed, a venous-arterial circuit was established with a stiff support wire, and atrial septal dilatation was performed with an 18 mm diameter balloon. We then premounted and crimped a 26 mm SAPIEN XT valve directly on the balloon of the NovaFlex delivery system to ensure that the prosthesis was well crimped in its delivery position and in order to avoid doing the usual loading of the device in the inferior vena cava. It is important to note that the valve is mounted on the delivery system in the opposite direction to that used for transfemoral retrograde aortic implantation. The atrial septum was then crossed and the SAPIEN XT positioned within the mitral bioprosthesis (slightly more atrially), and implanted during rapid pacing.
Study endpoints and definitions
We utilised the endpoint definitions of the Valve Academic Research Consortium (VARC) where available.25 Events were divided temporally into periprocedural, 30-day, and last clinical follow-up as appropriate. The following events were evaluated: vascular complications, bleeding, blood transfusions, acute kidney injury (AKI), cerebrovascular events including transient ischaemic attacks and stroke, myocardial infarction, death, and prosthetic valve performance. Vascular complications were divided into minor or major. Bleeding complications were divided into life threatening, major or minor bleeding. The AKI classification adopted the modified RIFLE (risk, injury, failure, loss, and end-stage kidney disease) criteria and includes three stages. VIV performance was evaluated immediately after implantation by quantifying the trans-prosthetic gradient along with the degree of regurgitation using echocardiography, aortography, and haemodynamic parameters. Repeat echocardiographic assessment was then performed at discharge, 30-days and 1-year. Finally, we evaluated the VARC composite endpoints of device success, the combined safety endpoint at 30-days (freedom from all cause mortality, major stroke, life-threatening bleeding, AKI stage 3, MI and major vascular complication), and the combined efficacy endpoint at the last clinical follow-up (freedom from all cause mortality after 30-days, failure of current AS therapy or prosthetic valve dysfunction). Clinical follow-up was performed by office visit at 30-days, 6-months and 1-year after the procedure or by telephone contact in those patients unable to return for clinical follow-up.
Statistical methods
Continuous variables are presented as means ±standard deviation or medians (interquartile range) and categorical variables as frequencies (%).
Results
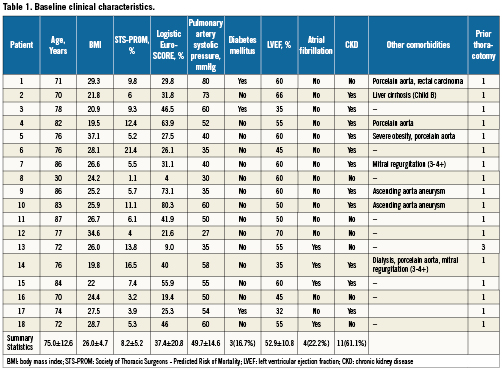
Baseline characteristics of the 18 patients who underwent transcatheter VIV (17 aortic and one mitral) are shown in Table 1. The mean age of patients (12 male, five female) was 75.0±12.6 years. This cohort of high-risk patients requiring redo valve replacement had a Society of Thoracic Surgeons predicted risk of operative mortality of 8.2±5.2% (Range: 1.1 to 21.4%) and a logistic EuroSCORE of 37.4±20.8% (Range 4-80.3%). Many patients had additional co morbidity which, irrespective of these risk scores, made them unfavourable candidates for conventional re-do surgery such as: severe pulmonary hypertension defined as a pulmonary artery systolic pressure ≥60 mmHg (n=5), porcelain aorta (n=4), concomitant severe mitral valve disease (n=2), aneurysm of the ascending aorta (n=2), malignancy requiring therapy (n=1), hepatic cirrhosis (n=1), multiple previous thoracotomies (n=1) and severe obesity (n=1).
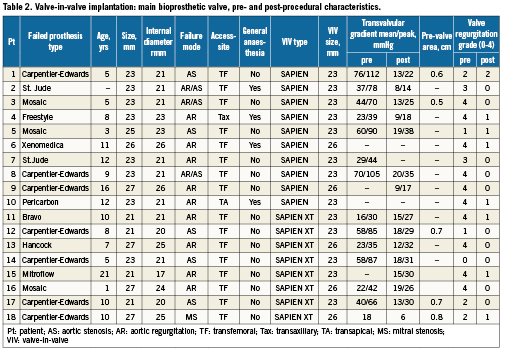
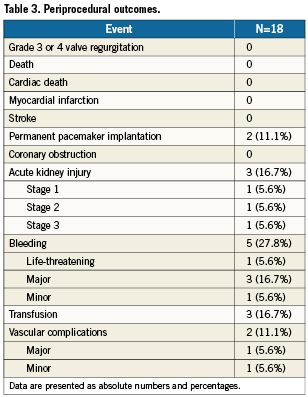
The average time from implantation to bioprosthetic failure was 8.5±3.8 years, with the mechanism of failure being regurgitation in nine, stenosis in six, and mixed regurgitation-stenosis in three. Characteristics of the failed bioprostheses and VIV procedure are shown in Table 2. Transfemoral VIV implantation was performed in all but two patients because of insufficient vessel diameter in one (transapical procedure) and the presence of an aorto-bifemoral bypass in the other (transaxillary procedure performed with Retroflex delivery system and Edwards SAPIEN valve).26 Predilatation of the bioprosthesis was required in two patients. In one patient with a significant post-THV paravalvular regurgitation (grade 3-4), postdilatation reduced the regurgitation to grade 2. VARC-defined device success was 94% with one patient having a post-implantation transaortic gradient ≥20 mmHg. The fluoroscopy time was 31.9±2.4 minutes and total contrast volume required for these procedures was 111.1±77.3 ml. Periprocedural outcomes are summarised in Table 3. There were no periprocedural or in-hospital deaths or neurological complications. Serious complications related to the transcatheter procedure included life-threatening or major bleeding (22.2%), major vascular complication (5.6%), and stage 3 acute kidney injury requiring haemodialysis (5.6%). In two of the four patients with life-threatening or major bleeding, this was related to a pre-existing condition: rectal carcinoma in one and Child-B cirrhosis with thrombocytopenia in the other. The length of the index hospitalisation was 8.3±6.8 days.
Valve performance as assessed by post-implantation aortography or pre-discharge echocardiography demonstrated a reduction in mean transvalvular gradient from 42.6±21.2 mmHg to 13.4±4.8 mmHg and no or grade 1 residual prosthetic regurgitation in 17 patients and grade 2 in one patient.
At 30-day clinical follow-up, all patients were alive and no patient had a suffered a cerebrovascular event or myocardial infarct. The safety of transcatheter VIV implantation in this cohort was 88.8% (16/18) at 30-days (VARC combined safety endpoint). At last clinical follow-up (median 11 months, interquartile range 6-17), the mortality rate was 5.6%. One patient died 386 days after the procedure due to a haemorrhagic stroke possibly related to an episode of endocarditis 6-months previously that was successfully treated with antibiotics. There was also another case of bacterial endocarditis 435 days after the procedure with a vegetation on the mitral valve and not on the THV, in a patient with known mitral valve disease and residual 2+ aortic regurgitation after the procedure. This case was also treated successfully with antibiotics. At last clinical follow-up, VIV implantation was efficacious in 83% (15/18) with three patients not meeting the VARC combined efficacy endpoint because of one death after 30 days, one re-hospitalisation for heart failure due to endocarditis, and one patient with a transaortic gradient ≥20 mmHg.
VIV implantation was very effective in reducing symptoms: 82% of patient were in NYHA class 3 or 4 before the procedure and all patients were in NYHA class II or lower at last clinical follow-up.
Discussion
The main findings of this case series of VIV implantation with the Edwards THV for a failed bioprosthesis are:
1) A transcatheter approach to VIV implantation in both the aortic and mitral positions is technically feasible via the transfemoral route, with a VARC-defined device success of 94%;
2) This approach was associated with excellent periprocedural and clinical outcomes, with a low mortality rate in a high-risk surgical group of patients, and
3) Favourable valve performance and symptomatic improvement was demonstrated at a median of 11-months follow-up.
The growing need for options to replace degenerated bioprosthetic heart valves in an aging patient population with increasing operative risk provided the impetus for this new indication for THV implantation. The VIV technique was first reported in a human in 2007, in a patient with a failed aortic bioprosthesis associated with severe regurgitation.15 In the last two years, a few case series, the majority with small numbers, have demonstrated encouraging results after VIV implantation with both commercially available devices (the Medtronic CoreValve and Edwards SAPIEN THV). The Medtronic CoreValve has been described as being implanted only in failed aortic bioprostheses, both via the transfemoral and transaxillary approach.15-18 The Edwards THV appears to have been implanted in a more versatile way in failed bioprostheses in the aortic, mitral, pulmonary and tricuspid positions.9,10,19,27-29 However, the majority of cases have been performed via the transapical and transatrial approaches.6,9,10,19,27 Indeed, the initial Edwards VIV aortic and mitral cases attempted by Dr. Webb and colleagues via the transfemoral approach were unsuccessful and complicated by embolisation of the THV.10 This led the authors to abandon this approach in favour of transapical placement, which was felt to provide greater stability and coaxial alignment during VIV implantation. Although the transapical approach offers a short, straight and highly predictable access to the degenerated prosthesis, it still requires a mini-thoracotomy and left ventricular apical access performed under general anaesthesia that in some high-risk patients may not be well tolerated and may increase post-procedural morbidity as well as hospital stay.
In this context, this case series is unique in that it demonstrates the feasibility of performing aortic VIV procedures with the Edwards SAPIEN THV via the less invasive transfemoral route with very high success rates and relatively few complications. Majority of procedures were performed under local anaesthesia and conscious sedation. The only major vascular complication in this series occurred when we were still using the larger bore Retroflex delivery system. A concerning finding in this case series are the two cases of infective endocarditis. However, both occurred more than one year after the procedure, highlighting the importance of antibiotic prophylaxis in these patients.30
There are certain technical aspects of VIV procedures that differ from native valve implantation which we would like to highlight. The choice of THV size (23 mm or 26 mm) was guided by the internal diameter for stented bioprostheses, whereas for stentless tissue valves we utilised the implanted valve diameter or annular diameter as measured on MSCT. In our experience, THV device size selection is different for VIV as compared to native valve procedures in that the THV external diameter does not have to always be larger than the bioprosthesis internal diameter and can even match the internal diameter, i.e., a 23 mm Edwards SAPIEN implanted in a bioprosthesis with a 23 mm internal diameter. The important factors that may make device sizing different in VIV procedures are that in comparison to native aortic valves, bioprostheses are: non- or only minimally distensible; provide almost always a nearly circular landing zone rather than the often oval-shaped native annulus; and have a sewing ring that provides excellent anchoring for VIV implantation.5 Nevertheless, we should acknowledge that the small numbers of cases treated are insufficient to establish firm recommendations. The majority (16/18) of the VIV procedures were performed without predilatation of the bioprosthesis. We attempted to avoid balloon valvuloplasty even if the prosthesis was stenosed due to concerns that balloon dilatation may disintegrate the valve and result in torrential regurgitation or embolisation of debris.5,16 However, it is essential to make an attempt to cross the valve with the valvuloplasty balloon prior to insertion of the Edwards delivery system, which, in its current version, cannot be retrieved without implanting the valve. The reason that predilatation may be deleterious for THV implantation in a failed bioprosthesis could be related to the fact that the pathology and mechanisms of failure within a bioprosthesis are quite different from the calcific degeneration seen in native valve aortic stenosis. Generally, bioprosthetic failure can result from calcification, pannus, wear and tear, thrombosis, and/or endocarditis.5 Although calcific degeneration is common to both degenerative native and bioprosthetic valves, pannus (a host tissue response) is unique and inflammation appears to be more striking with bioprosthetic valve degeneration.5,31
Embolisation of the THV during VIV procedures has been reported and been thought to occur due to a lack of coaxiality during implantation or a suboptimal deployment position. Incorrect positioning of the THV on the outflows of the surgical prosthesis may result in splaying of the prosthetic struts and THV embolisation.10 Thus, although the bioprosthesis often provides a highly visible radiographic target for VIV placement, it is important that the operator is familiar with the anatomy and structure of the failed bioprosthesis. In particular, it is essential to identify the location of the sewing ring, as overlapping of this ring during VIV implantation should prevent valve embolisation. In cases of stentless valves or non-radiopaque stented valves such as the St Jude valve, aortography was helpful in selecting the appropriate implantation position. Transoesophageal echocardiography may also be useful in guiding VIV placement.
Coaxial alignment has been suggested to be an important factor in VIV implantation.6,10 Although we always attempted to coaxially align the THV within the failed bioprosthesis, this was not always possible. We found that ensuring complete coaxiality may not always be necessary because this step occurs almost spontaneously at the time of slow balloon inflation during rapid pacing. Although there have been recent reports of coronary obstruction during VIV procedures with the Mitroflow® (Sorin SpA, Milan, Italy) prosthesis32, we did not observe any in this case series and have also treated one failed Mitroflow valve which differs from other stented bioprostheses as the leaflet tissue is mounted externally over the stent as opposed to internally. Finally, an important unanswered question is whether the durability of a THV will be different if placed within a bioprosthesis as compared to a native valve. Indeed, expansion of the THV may be different but the long-term impact of this on valve performance is still unknown.6
Conclusions
Transcatheter implantation of the Edwards THV within a degenerated aortic bioprosthesis, and performed predominantly via the transfemoral route, is feasible and associated with good periprocedural and clinical outcomes in high-risk surgical patients. As continued data becomes available confirming the reproducibility and durability of these procedures, we might see a paradigm shift in surgical valve replacement with more patients being treated with bioprosthetic valves and percutaneous VIV procedures being preferred over conventional redo valve surgery as the primary approach for tissue valve degeneration.
Conflict of interest statement
M. Montorfano is a Proctor for Edwards Lifesciences Inc. F. Maisano is a Consultant for Medtronic Inc. None of the other authors have any conflicts of interest to declare.

