
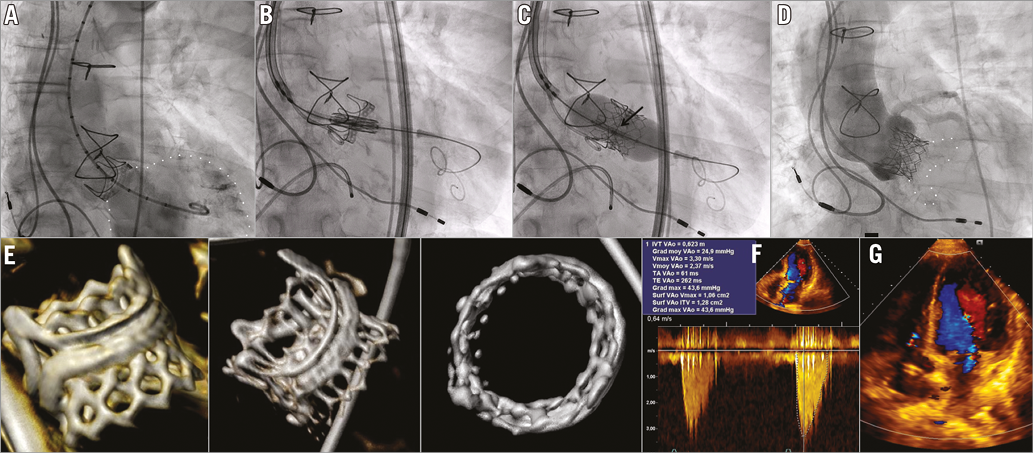
An 87-year-old female was admitted for symptomatic failure of a 23 mm Carpentier-Edwards PERIMOUNT aortic bioprosthesis (Edwards Lifesciences, Irvine, CA, USA) implanted 17 years previously. Both severe central regurgitation and severe stenosis (mean gradient 60 mmHg) were identified. Due to a significant perioperative risk of redo surgery (EuroSCORE 46.5%, STS 7.3%), the Heart Team decided on transfemoral valve-in-valve (ViV) implantation. A 23 mm Edwards SAPIEN 3 (S3) device (Edwards Lifesciences) was chosen, based on a measured inner diameter of 20.5 mm by multislice computed tomography (MSCT) and of 21 mm as reported by the manufacturer. The ViV procedure was performed under conscious sedation and fluoroscopy guidance. The central radiopaque marker of the S3 was positioned 1 mm above the basal ring of the PERIMOUNT, aligning the top of the S3 with the top of the failing bioprosthesis. Adequate anchoring was achieved without any coronary obstruction and trivial periprosthetic regurgitation (panel A-panel D, Moving image 1-Moving image 5). Post-procedure MSCT confirmed the proper placement of the S3 within the failed PERIMOUNT (panel E). Discharge mean transprosthetic gradient was 25 mmHg (panel F, panel G). Two months later, the patient was in NYHA Class II and the mean transprosthetic gradient had dropped to 15 mmHg.
Post-procedural mean gradients greater than 20 mmHg remain frequent in ViV for failed bioprostheses with inner diameters smaller than 21 mm. The external cuff of the S3 could provide additional constraints to the stent frame within the failed device generating high gradients. A SAPIEN XT transcatheter heart valve (Edwards Lifesciences) may still be appropriate. However, an accurate positioning will be facilitated by the S3 central marker.
Conflict of interest statement
D. Tchetche is proctor for Edwards Lifesciences, Medtronic and Boston Scientific. The other authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Ascendant aortic angiogram showing severe aortic regurgitation with a pigtail drop into the left ventricle.
Moving image 2. Angiographic view of S3 positioning.
Moving image 3. Angiographic view of S3 deployment under rapid ventricular pacing.
Moving image 4. Angiographic view of S3 post dilatation.
Moving image 5. Final ViV angiographic result with trivial periprosthetic regurgitation.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Ascendant aortic angiogram showing severe aortic regurgitation with a pigtail drop into the left ventricle.
Moving image 2. Angiographic view of S3 positioning.
Moving image 3. Angiographic view of S3 deployment under rapid ventricular pacing.
Moving image 4. Angiographic view of S3 post dilatation.
Moving image 5. Final ViV angiographic result with trivial periprosthetic regurgitation.

