CASE SUMMARY
BACKGROUND: A 64-year-old female with rheumatic heart disease and multiple prior valve replacements presentedwith progressive oedema, ascites and dyspnoea on exertion.
INVESTIGATION: Physical examination, transthoracic echocardiography, intracardiac echocardiography, transoesophageal echocardiography, right heart cathetherisation, computed tomography.
DIAGNOSIS: She had a mitral homograft and Physio ring in the tricuspid position, and presented with severe bioprosthetic tricuspid valve stenosis (mean gradient 16 mmHg) and right-sided heart failure.
TREATMENT: A transcatheter 26 mm Edwards SAPIEN valve was placed in the tricuspid position, resulting in near normalisation of tricuspid valve gradient. This represents the first report of a combined valve-in-ring (VIR) and valve in a homograft valve (VIV) SAPIEN implantation.
KEYWORDS: SAPIEN, structural heart interventions, transcatheter aortic valve replacement, valve-in-valve, valvular heart disease
PRESENTATION OF THE CASE
The patient is a 64-year-old female with a history of rheumatic heart disease. She had four prior sternotomies for valve replacement and most recently underwent mechanical mitral valve replacement, mechanical aortic valve replacement (both St. Jude Medical, St. Paul, MN, USA), and placement of a mitral valve homograft with a #34 Physio ring (Edwards Lifesciences, Irvine CA, USA) in the tricuspid position. She is pacemaker dependent. Over the preceding months she had been experiencing progressive congestive hepatopathy requiring dieresis and paracentesis with oedema, ascites, and dyspnoea on exertion, and was admitted with NYHA Class IV symptoms. Echocardiography revealed severe tricuspid stenosis with a mean gradient of 16 mmHg and tricuspid valve area of 0.5 cm2, with mild tricuspid regurgitation. She was initially referred for surgical tricuspid prosthesis explant and replacement. How should severe prosthetic tricuspid stenosis in an extreme surgical risk patient be treated?
How would I treat?
THE INVITED EXPERTS’ OPINION
The first step of the management strategy in this patient should be to eliminate, using echocardiography, dysfunction of either the aortic or mitral prosthesis, and also very severe left or right ventricular dysfunction. If none of these is the case then stenosis of the tricuspid homograft is most likely responsible for the clinical condition and will need to be treated. The “heart team”1 should evaluate:
1. the life expectancy of the patient taking into account the comorbidities;
2. the risks of redo surgery due to the general condition of the patient and the anticipated technical difficulty related to the multiple previous cardiac interventions.
If the life expectancy is acceptable and surgery is confirmed to be at very high risk, which seems to be the case at first glance, percutaneous tricuspid valve-in-a-valve replacement (PTVR) should be considered. PTVR for tricuspid bioprosthetic failure has been described previously2,3. However, there is no report on PTVR in a mitral homograft in the tricuspid position. On the other hand, this patient has a surgical ring in the tricuspid position, and percutaneous valve-in-ring in mitral position has been reported4, as has percutaneous tricuspid valve-in-ring through a surgical transatrial approach5. Following these examples PTVR could be attempted using either a transatrial, a transjugular or a transfemoral approach, the latter being our preferred option2. After an integrative approach using 3-D echocardiography, MSCT, and fluoroscopy for the measurement of the tricuspid annulus internal size, a SAPIEN XT 29 mm (Edwards Lifesciences, Irvine, CA, USA) prosthesis is likely to be used in this 34 mm Physio surgical ring. Finally, the pacemaker already in place can be used for the rapid pacing during PTVR and the pacing lead would likely remain in place between the SAPIEN prosthesis and the surgical ring after implantation. Mild to moderate residual paravalvular TR may occur related to the presence of the lead but this will not have the same negative consequences as it would in the mitral position (risk of haemolysis). In case of dysfunction of the pacemaker a lead should be implanted via the coronary sinus2 allowing temporary pacing whilst waiting for another permanent implant.
Conflict of interest statement
A. Vahanian received speaker’s honoraria from Edwards Lifesciences and is a consultant (Advisory Board) for Abbott, Medtronic, St. Jude Medical, Valtech. D. Himbert is a proctor for Edwards Lifesciences and Medtronic Inc. The other authors have no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERTS’ OPINION
The indication for intervention in this case is clear. Considering the significant risk associated with surgical reintervention6, we propose transcatheter tricuspid valve replacement. Initial experience with this approach for failed surgical bioprostheses was described by Webb et al via a transatrial approach7. Further reports followed describing transfemoral and transjugular approaches8-10. Case reports have shown good outcomes in patients who are high risk for surgery, although concerns regarding early valve failure have been published11.
The more complex issue considering the patient’s pacemaker dependence is how to deal with the right ventricular pacing lead. Transcatheter valve implantation would necessitate jailing the existing lead, which could jeopardise lead integrity and function. Lead extraction is often a barbaric process with major complications reported in 5%12. If the lead was removed prior to the procedure, a temporary lead across the tricuspid valve would also be jailed by the transcatheter valve. A coronary sinus lead may prove very challenging to place in the setting of such a dilated right atrium.
Having considered all these options and discussed the case with our EP and surgical colleagues, we propose implantation of an epicardial VVI system through a small subxiphoid incision in a hybrid lab setting. This would allow for pacing during the procedure should the right ventricular endocardial lead become compromised. We would then perform the procedure through the right femoral vein as the pacing leads in the superior vena cava may complicate an internal jugular approach, although it has been suggested the neck provides a more favourable route to the tricuspid valve. Although the internal diameter of the tricuspid complex by CT scan is 26.6 mm, it is likely that the functional diameter is significantly less. Proper sizing of the functional annulus should be performed with transoesophageal echocardiography and balloon sizing with a compliant balloon and accurate assessment of the waist size prior to commitment to the final valve size. Wire position at this point should be in the right pulmonary artery and, although there are advocates for a right ventricular apical position, we feel wire stability is more of a concern with this approach. Valve choice may influence the need for pre-stenting with reports suggesting pre-stenting is not necessary with the Melody® valve (Medtronic Inc., Minneapolis, MN, USA)8. However, previous reports have described transcatheter valve placement within a stented bioprosthesis, and it is unclear how much support will be provided by a mitral homograft. Our approach would be pre-stenting with a P3110 stent (Cordis, Johnson & Johnson, Warren, NJ, USA) on a 24 mm BIB balloon (NuMed, Hopkinton, NY, USA) catheter followed by placement of a 26 mm Edwards SAPIEN valve. The outer diameter of a Melody valve on a 24 mm BIB is also just over 26 mm so there may not be too much difference between these from a sizing perspective. The Edwards 29 mm valve has also been reported in the tricuspid position but this may not be available in the USA outside of clinical trial usage13.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
After discussion with the patient, referring physician, and cardiothoracic surgery, it was collectively felt that her surgical risk was nearly prohibitive, and a percutaneous approach was recommended. We performed the procedure under general anaesthesia and TEE guidance. Access was obtained in the right internal jugular vein in the event of need for emergent rescue pacing, left femoral artery for continuous arterial pressure monitoring, and in the right femoral vein with a “pre-close” technique utilising two Perclose® devices (Abbott Vascular, Santa Clara, CA, USA). A 14 Fr sheath was advanced through the right femoral vein into the inferior vena cava, and the balloon tip of a 7 Fr Arrow wedge catheter (Arrow International, Diegem, Belgium) was advanced through the prosthetic valve into the right ventricle. The Arrow catheter was then exchanged for a 6 Fr pigtail catheter over a 0.032” Amplatz Extra-Stiff (AES) wire (Cook Medical, Bloomington, IN, USA) and right ventriculography was performed. Next, a Z-Med II - 23 mm×6 cm balloon (B. Braun Interventional Systems, Bethlehem, PA, USA) was advanced over the AES wire, positioned across the tricuspid valve, and balloon valvuloplasty performed (Figure 2A). The Z-Med balloon was removed, and the 14 Fr sheath was exchanged for the Edwards RetroFlex 3 delivery system (Edwards Lifesciences). An IntraStent® LD Max™ – 12 mm×26 mm (Covidien, Mansfield, MA, USA) was loaded and crimped on a BIB balloon (Numed, Denton, TX, USA) and advanced over the AES wire, and positioned across the tricuspid valve. The stent was deployed in stepwise fashion (inner balloon at 4.5 atmospheres, outer balloon at 3 atmospheres) (Figure 2B). Next, a #26 SAPIEN valve was prepared and crimped in the usual fashion, inserted through the sheath, and positioned across the tricuspid apparatus within the IntraStent, and deployed with rapid ventricular pacing under fluoroscopic and transoesophageal echocardiographic (TEE) guidance (Figure 2C and Figure 2D). Rapid pacing was performed using the patient’s internal RV lead and device with the programmer. Subsequent ventriculography, haemodynamic and echocardiographic assessment revealed trivial tricuspid regurgitation and a mean gradient of 3 mmHg across the tricuspid valve apparatus (Figure 2E and Figure 2F). Heparin was used for anticoagulation during the procedure.
The patient tolerated the procedure well, was extubated the day of the procedure and transferred in stable condition to the cardiac care unit. On post-procedural day five, she suffered a spontaneous left retroperitoneal bleed in the setting of an elevated partial thromboplastin time. Follow-up echocardiogram one week after the procedure revealed a stable gradient, no tricuspid regurgitation, and no pericardial effusion.
While anecdotal reports of Melody or SAPIEN valve implantation within tricuspid bioprostheses have been reported, the present case of SAPIEN VIR and VIV (mitral homograft) implantation is unique for a number of reasons3,8,9. SAPIEN VIR in the mitral position has been performed in humans via the transapical route14, and Melody VIR has been performed via the transseptal approach in animal models15. In the present case, the Physio ring (initially designed for the mitral annulus) is a complete rigid ring with the theoretical advantage of better prosthesis apposition, and an accordingly reduced risk of device embolisation or paravalvular leak8,15.
Prosthesis sizing is an essential pre-procedural consideration for VIR and VIV procedures. The native tricuspid valve annulus is frequently ovoid with major and minor diameters that differ only marginally during systole16. We estimated the tricuspid prosthesis size by computed tomography, intracardiac echocardiography, and finally by balloon sizing during the procedure (Figure 1, Figure 2A). The dimensions of the tricuspid valve annulus are better approximated by the commercially available SAPIEN valves (up to 26 mm in diameter) than the Melody valves (maximum expandable diameter 22 mm)16-18. In fact, expansion of Melody valves to larger diameters has been associated with valvular incompetence and repeated device embolisation15.
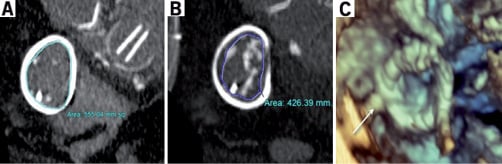
Figure 1. A) Computed tomogram of the tricuspid ring and mitral valve prosthesis is visible. The area of the Physio ring was evaluated in this view, and the mitral valve homograft skirt was also estimated. B) The ring and homograft area estimates corresponded to mean diameters of 26.6 mm and 23.3 mm, respectively. C) 3-dimsional transesophageal echocardiography depicting an atrial view of the tricuspid prosthesis

Figure 2. SAPIEN valve-in-valve and valve-in-ring implantation for tricuspid stenosis. A) Z-Med 23 mm×6 cm balloon across the tricuspid apparatus for valvuloplasty and to assist in prosthesis sizing. B) Deployed IntraStent through the Edwards sheath. C) Right ventriculogram after SAPIEN deployment within IntraStent. D) Lateral view of expanded SAPIEN valve within mitral homograft and Physio ring. E) Tricuspid valve gradient measured with dual lumen catheter before, and after (F) SAPIEN valve implantation.
Pre-stenting is typically performed when Melody valves are implanted in the pulmonic position to create a stable landing zone and to reduce the risk of stent fracture. Although there have been no reported cases of SAPIEN valve fracture in the considerable published TAVR experience, we chose to use a pre-stenting technique for two reasons: to ensure coverage of the valvular orifice and to enable better valve positioning. In contrast to aortic VIV procedures where the prosthesis cage can facilitate valve positioning with simple fluoroscopy, the Physio ring annulus does not accurately represent the plane of the apically displaced valve orifice. We also considered the relative rigidity of the Edwards delivery system compared to the Melody valve delivery system, and the fact that a stable landing zone may facilitate coaxial positioning. Importantly, despite the combination of a #26 SAPIEN valve (16.1 mm height at full deployment) within the IntraStent (26 mm pre-deployment), we did not overlap with the right ventricular outflow tract – a consideration with pre-stenting in available series (Figure 2C)8.
Although tricuspid VIV procedures have been performed via both the internal jugular and femoral venous routes, we selected the latter based on a relatively horizontal orientation of the valve, and on our familiarity with the working angles enabled by the Edwards RetroFlex delivery sheath. We considered the existence of the pacemaker lead coursing through the prosthetic tricuspid valve, the potential risk of procedural lead fracture or dislodgement, and relative risk and invasiveness of alternatives (extraction, placement of a subcutaneous pacemaker). We elected to deploy over the right ventricular lead, entrapping it against the annulus as previously described19, but with the precaution of internal jugular access in event of the need for emergent pacing. Follow-up interrogation of the pacemaker revealed stable thresholds and impedance.
A final consideration in all percutaneous structural heart interventions is the optimal imaging modality. Pre-procedural CT was performed to estimate the TV annulus size and intraprocedural TEE was used to monitor for interval development of valvular incompetence. Available registries have uniformly employed TEE, although intracardiac echocardiography, particularly with emerging three-dimensional capability, might be a suitable alternative (Figure 1C).
Conflict of interest statement
S. Lilly receives research funding to his institution from Edwards Lifesciences to participate in the Partner trials. The other authors have no conflicts of interest to declare.

