CASE SUMMARY
BACKGROUND: An 83-year-old woman was referred to our dedicated out-patient clinic for evaluation of the feasibility of transcatheter mitral valve-in-valve implantation. She was heavily symptomatic for dyspnoea, and was not a candidate for conventional surgery (logistic EuroSCORE 54.27%). The pre-procedural evaluation confirmed the presence of a severely degenerated 27 mm Carpentier-Edwards porcine mitral bioprosthesis, and revealed the presence of a severely degenerated, malfunctioning 21 mm Sorin Mitroflow aortic bioprosthesis.
INVESTIGATION: Clinical assessment, echocardiography, cardiac catheterisation including coronary angiogram and aortography during balloon aortic valvuloplasty (BAV), cardiac CT.
DIAGNOSIS: Structural valve degeneration of the mitral bioprosthesis causing severe mitral stenosis and moderate to severe mitral regurgitation. Structural valve degeneration of the aortic bioprosthesis causing severe aortic stenosis and severe aortic regurgitation. Tricuspid regurgitation. Pulmonary hypertension. Chronic renal failure. Patency of the coronary arteries during BAV.
TREATMENT: Staged transapical mitral valve-in-valve implantation with a 26 mm Edwards SAPIEN valve and percutaneous aortic valve-in-valve implantation with a 23 mm Medtronic CoreValve.
KEYWORDS: aortic valve disease, mitral valve disease, transcathether aortic valve implantation
PRESENTATION OF THE CASE
An 83-year-old woman with a malfunctioning mitral bioprosthesis was referred to our dedicated out-patient clinic for evaluation of the feasibility of transcatheter mitral valve-in-valve implantation. In 2000 the patient had undergone triple valve surgery: mitral valve replacement with a 27 mm Carpentier-Edwards porcine valve (Edwards Lifesciences, Irvine, CA, USA), aortic valve replacement with a 21 mm Mitroflow® valve (Sorin Group, Milan, Italy) and De Vega tricuspid annuloplasty. She had chronic renal failure, and was heavily symptomatic for dyspnoea. Transthoracic echocardiography confirmed the presence of a marked structural deterioration of the mitral bioprosthesis, causing severe stenosis with a mean transprosthetic gradient of 17 mmHg, and moderate to severe regurgitation. The examination also revealed the presence of a severe structural degeneration of the aortic bioprosthesis: the mean transprosthetic gradient was 48 mmHg, the aortic valve effective orifice area was 0.5 with 3+/4+ aortic regurgitation. The patient had severe pulmonary hypertension (pulmonary artery systolic pressure was 70 mmHg) and moderate to severe tricuspid regurgitation. The left and right ventricular function was normal. The logistic EuroSCORE I was 54.27%, due to the following: female gender, creatinine >2, pulmonary hypertension, previous cardiac surgery, and “other than isolated CABG”. The STS score was not available for a double valve procedure. The STS score for the mitral procedure alone was 16.43%. Despite the fact that the patient appeared undeniably fragile, she was still active and independent, and desired to be treated and to return to the normal life that she had conducted until recently.
The patient was brought to the attention of our institution’s TAVI team and, although all the team members had the perception that she deserved to be treated, the risk of a conventional surgical procedure was judged unacceptable. On the other hand, the presence of a severely degenerated, malfunctioning 21 mm Mitroflow bioprosthesis in the aortic position dampened the enthusiasm for a double valve-in-valve procedure. In fact, the nominal internal diameter of this surgical valve, as provided by the manufacturer, is 17.3 mm: this would expose the patient to a significant risk of underexpansion of the transcatheter prosthesis, and to a high probability of significant post-procedural patient-prosthesis mismatch1. Moreover, there is a known risk of coronary ostial occlusion when performing a valve-in-valve procedure inside a Mitroflow bioprosthesis2.
Should this lady be considered for optimal medical therapy? Or should the risk of conventional surgery be accepted, considering the high degree of independence and the good performance status that the patient had experienced until recently? Or, finally, should transcatheter therapy be attempted?
How would I treat?
THE INVITED EXPERTS’ OPINION
This case presents a clinical dilemma and we share concerns similar to those of the authors. In the balance is the very high risk of the procedure due to comorbidities with a EuroSCORE of 54.27% against very poor prognosis with medical treatment3. There are three important decisions to be made:
1. Whether valve-in-valve (VIV) is appropriate?
2. If yes, then which route?
3. What sequence of implantation?
Considering the patient is 83 years old and was independent up until a few weeks before presentation makes us err towards performing a valve-in-valve (VIV) procedure. Further, both the valves are stenotic and regurgitant, and hence neither medical management nor balloon valvuloplasty will relieve the patient of her symptoms and in fact may worsen them4-6. VIV is the only viable option available for her treatment.
We would use the SAPIEN XT (Edwards Lifesciences) as the device for both aortic and mitral VIV but the CoreValve® Evolut™ (Medtronic Inc., Minneapolis, MN, USA) could be used for the aortic position4,5. To reduce the procedural complications and improve the chances of success, we would propose the following two options for this patient.
Depending on the suitability of the femoral arteries, two options are available, namely:
Option 1 (preferred)
If the femoral arteries are suitable (>6 mm, with minimal calcification, angulation and tortuosity) for a 23 mm SAPIEN XT implant then we would perform a transfemoral (TF) VIV with a 23 mm SAPIEN XT using the NovoFlex+ Transfemoral System (Edwards Lifesciences).
We would place guidewires in the coronary ostia if the aortic root is narrow to provide access to them in case of obstruction. The coronary obstruction incidence is <3% but the feasibility of using a 23 mm SAPIEN XT to treat a 21 mm Mitroflow has been reported3,4. Following this, an antegrade VIV procedure for the mitral valve using a NovoFlex+ and a 26 mm SAPIEN XT should be performed through a transseptal puncture utilising the femoral venous access. The feasibility of this approach has also been demonstrated7,8.
As both the valve sizes are known and are visible under fluoroscopy, selection of the SAPIEN XT as per the true internal diameter (ID) and placement in the correct position can be performed using available guidance9,10. The patient does not require preoperative cardiac CT with the associated risk of renal injury or intraoperative echo, and the procedure can be performed under sedation with fluoroscopic guidance alone4,10.
Option 2
If the femoral arteries are not suitable for a 23 mm SAPIEN XT then a double VIV procedure should be performed through the transapical (TA) approach using the Ascendra+ System (Edwards Lifesciences). The procedure would be performed under general anaesthesia, but care should be taken to minimise trauma from rib spreading and to provide excellent analgesia postoperatively11. The aortic bioprosthesis should be treated first with a 23 mm SAPIEN XT using the same technique as above, followed by placement of a 26 mm SAPIEN XT in the mitral position. This sequence allows easier placement of an aortic VIV, as crossing the aortic valve through the LVOT is challenging once mitral VIV has been performed12.
There is a risk of high post-procedure gradients in the aortic position but, considering her level of activity, eliminating regurgitation should be clinically beneficial as observed in large series3,4,11.
Conflict of interest statement
V. Bapat is a consultant for Edwards Lifesciences, Medtronic Inc., and St. Jude Medical. R. Attia has no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERTS’ OPINION
A cardiac CT scan would be required before deciding upon the strategy in order to show the feasibility of transfemoral TAVI and the distance between the valve and the coronary arteries and the size of the Valsalva sinuses.
The final decision is usually that of the “Heart Team” in such a patient who is not sufficiently represented in the sample population from which any surgical risk score assessment was built. Since the patient seems to be active and willing to undergo a procedure, an intervention should be considered.
According to the description, surgery seems to be very high risk; thus, TAVI is the only alternative to medical therapy.
Performing valve-in-valve implantation in aortic Mitroflow bioprostheses is challenging and risky3. However, it may be considered if TAVI is the only option and if the height of the coronary arteries is sufficient and/or if the Valsalva sinuses are not too small. A useful additional step could be to perform aortography during balloon aortic valvuloplasty (BAV) in order to evaluate the risk of coronary occlusion. Performing BAV in a degenerated left-sided bioprosthesis should usually be avoided but, in such a case, the risk-benefit ratio could plead in favour of this procedure.
There are two options for the approach for TAVI:
The first one is the transapical approach (TA), which would allow treatment of both valves. However, it would be regrettable to pursue this approach if the procedure is stopped after BAV. In addition, it carries the risk of the inherent complications in such a fragile patient and the suboptimal haemodynamic performance of balloon-expandable prostheses, which are necessarily used during TA.
The alternative would be first to perform the transseptal puncture, leaving the sheath in the left atrium, and then to perform transfemoral implantation of the aortic valve. A small CoreValve (23 mm, Medtronic) would probably be the ideal option, firstly because it could be retrieved during deployment if hypotension occurs suggesting the possibility of coronary occlusion, and secondly because the supravalvular valve function is likely to provide better final haemodynamics than a balloon-expandable valve13. No doubt the residual gradient will be higher than expected but this would certainly be sufficient in such a patient.
If the aortic procedure is successful, a transseptal mitral “valve-in-valve” procedure could then be performed14. A balloon-expandable prosthesis could be used after careful sizing of the inner diameter of the valve. At first glance a 26 mm SAPIEN XT would probably fit.
Our preference would be for the second option. The TA approach is still valid and may even be the only option if the diameter of the femoral arteries contraindicates a femoral approach.
Conflict of interest statement
D. Himbert is a consultant and proctor for Edwards Lifesciences and Medtronic Inc. A. Vahanian is a consultant for Abbott Vascular, Medtronic Inc., and Edwards Lifesciences. The other authors have no conflicts of interest to declare.
How did I treat?
The Mitroflow aortic bioprosthesis (Sorin Group) has a peculiar design in that the pericardial leaflets are mounted outside the valve stent. As a consequence, they can be displaced towards the coronary ostia during the deployment of transcatheter valves. This might explain the exceptionally high rate of coronary ostial occlusion observed following valve-in-valve implantation in this particular bioprosthesis2,3. The first step in the management of our patient was therefore the evaluation of the risk of coronary artery occlusion. This was obtained by performing an aortography during balloon aortic valvuloplasty (BAV). We have previously described this approach in detail15. The procedure was performed with an 18 mm valvuloplasty balloon (Cristal balloon; Balt, Montmorency, France). The appearance of an evident waist on the balloon proved that the internal diameter of the surgical prosthesis was smaller than 18 mm, and the aortography, performed at full balloon inflation, demonstrated the patency of the coronary arteries (Figure 1, Moving image 1). The procedure confirmed that the malfunctioning bioprostheses could safely accommodate a transcatheter valve. It also led to a significant reduction of the aortic transprosthetic gradient to 30 mmHg, while leaving the degree of aortic regurgitation unchanged.
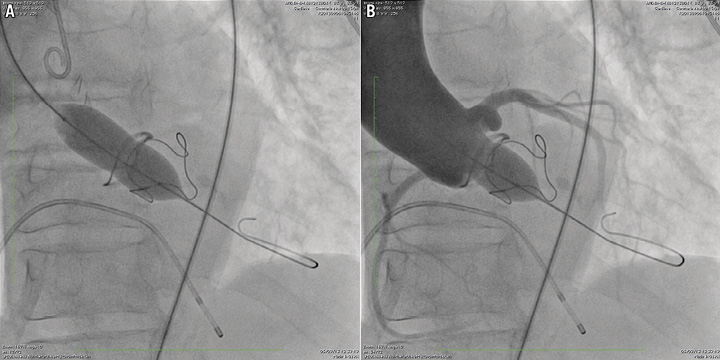
Figure 1. Aortography during balloon aortic valvuloplasty.. Balloon aortic valvuloplasty was performed to obtain an accurate measure of the surgical prosthesis internal diameter, and to obtain an estimate of the risk of coronary ostial occlusion. The procedure was performed with an 18 mm Cristal balloon, and demonstrated that the prosthesis internal diameter was less than 18 mm (A), and that the displacement of leaflets did not occlude the coronary ostia (B).
At this stage, several strategies were still available. Simultaneous transapical double valve-in-valve implantation with the Edwards SAPIEN valve has been successfully performed by several groups3, and appeared to be the simplest and most straightforward option. However, we were concerned that implanting a 23 mm SAPIEN in a 21 mm Mitroflow with an internal diameter smaller than 18 mm could have resulted in incomplete stent expansion and severe prosthesis-patient mismatch.
This problem could have been partially overcome by implanting a 20 mm SAPIEN in the aortic position. This approach has recently been used with acceptable results (Dr Danny Dvir, personal communication). However, the 20 mm Edwards SAPIEN valve is not presently available on the Italian market, and the experience with this particular device is limited.
Recently, the results from the Global Valve in Valve Registry3 have shown that almost 12% of patients with small bioprostheses (internal diameter <20 mm) receiving an Edwards SAPIEN valve develop a significant transprosthetic gradient of more than 40 mmHg, as opposed to 0% of patients receiving a Medtronic CoreValve. This difference is probably explained by the supra-annular design of the CoreValve. For this reason, we considered percutaneous implantation of a 23 mm Medtronic CoreValve as the procedure of choice to treat the degenerated aortic bioprosthesis in our patient.
Simultaneous percutaneous transfemoral aortic valve-in-valve and transvenous transseptal mitral valve-in-valve implantation was considered. However, due to the close spatial relationship between the mitral and aortic bioprostheses, we deemed it essential to centre the transcatheter valve in the mitral position as accurately as possible in this particular patient. The positioning of the transcatheter valve has been reported to be less predictable through the percutaneous transseptal route11,16. We therefore decided to treat the severely degenerated mitral bioprosthesis first, and assigned the patient to transapical mitral valve-in-valve implantation. The procedure, which has previously been described in detail elsewhere17, was performed as usual. The postoperative course was complicated by minor bleeding from the chest drainage insertion site, and the patient was discharged on postoperative day 10. At discharge, the mean transprosthetic gradient (mitral position) was 5 mmHg, and there was no residual mitral regurgitation.
At one month after the operation, the patient was re-assessed at our out-patient clinic. The echocardiography confirmed the positive outcome of the mitral procedure, and demonstrated the presence of severe aortic stenosis (the mean transprosthetic gradient in the aortic position was 52 mmHg, close to the baseline level) and moderate to severe aortic regurgitation. The patient was still symptomatic, and was readmitted for transcatheter aortic valve-in-valve implantation. A non-enhanced, ECG-gated CT scan was performed to evaluate the spatial relationship between the aortic bioprosthesis and the mitral transcatheter valve which protruded in the left ventricular outflow tract (Figure 2). The CT scan showed that the minimal distance between the Sorin Mitroflow sewing ring and the Edwards SAPIEN XT metal stent was 6 mm. Under fluoroscopy, a 23 mm Medtronic CoreValve was advanced in the left ventricular outflow tract through the right femoral artery, and leaned on the Edwards SAPIEN prosthesis stent. It was then released while gently pulling it towards the aorta (Figure 3). Due to the presence of a paravalvular leak causing moderate aortic regurgitation (Moving image 2), the valve was post-dilated with an 18 mm valvuloplasty balloon (Cristal balloon; Balt, Montmorency, France). The procedure was uncomplicated, and the patient was discharged on postoperative day seven. At discharge, the echocardiography confirmed the excellent result of the mitral procedure (mean gradient 5 mmHg and no regurgitation), and demonstrated the presence of moderate aortic prosthesis-patient mismatch (the mean transprosthetic gradient was 28 mmHg, with trace aortic regurgitation). At the two-month follow-up visit, the patient was asymptomatic for dyspnoea, and her general condition had improved significantly. The mean transprosthetic gradient across the mitral prosthesis was 5 mmHg, with no regurgitation. The mean transprosthetic gradient across the aortic prosthesis was 25 mmHg, with trace AR.
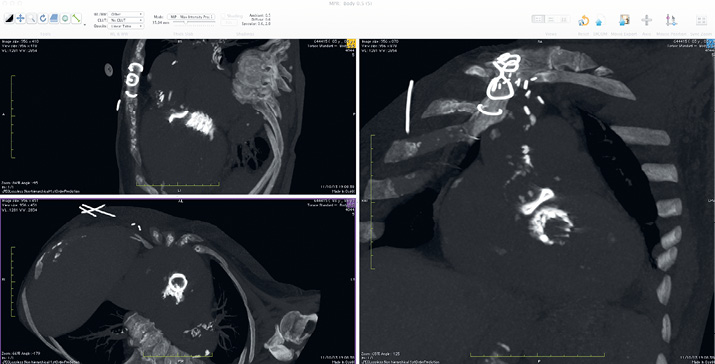
Figure 2. Chest CT, 3D MPR. A non-enhanced, ECG-gated chest CT was performed to evaluate the spatial relationships between the aortic Mitroflow bioprosthesis and the transcatheter valve in the mitral position. The minimal distance between the prosthesis sewing ring and the SAPIEN XT metal stent was 6 mm, leaving enough space below the aortic annulus for anchoring a Medtronic CoreValve transcatheter prosthesis.
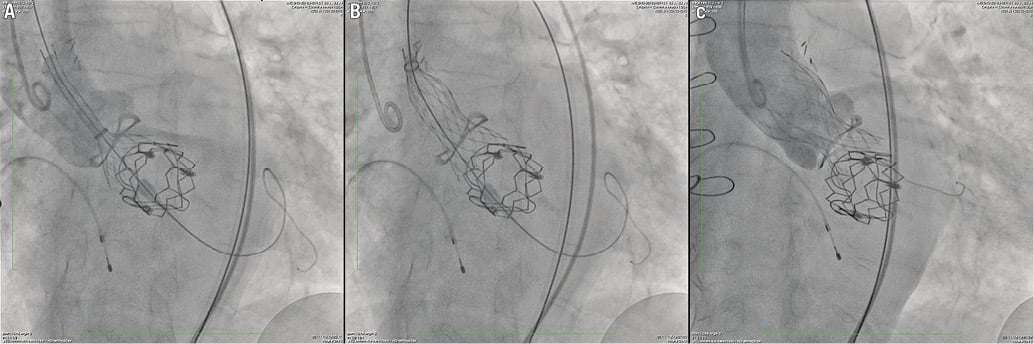
Figure 3. Aortic valve-in-valve implantation. The Medtronic CoreValve was leaned on the Edwards SAPIEN stent protruding in the left ventricular outflow tract (A), and slowly released while gently pulling it (B). Final result (C). The haemodynamic gradient (peak to peak) was 20 mmHg and there was mild intervalvular regurgitation.
Discussion
Transcatheter valve-in-valve implantation is an excellent option for high-risk patients with degenerated surgical bioprostheses and can lead to significant symptomatic improvement also in complex cases such as the one presented here. The success of the procedure is highly dependent on accurate planning and on the choice of the right device(s) and access route(s) for every interventional target. All the available knowledge and technologies should be used to achieve an optimal result.
Conflict of interest statement
S. Berti is a proctor for Edwards Lifesciences. U. Schäfer is a proctor and medical consultant for Medtronic Inc. and Edwards Lifesciences. The other authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. The aortography, performed at full balloon inflation, demonstrates the patency of both the coronary arteries.
Moving image 2. The CoreValve has been released, and the aortography demonstrates the presence of mild residual aortic regurgitation.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1.
Moving image 2.

