
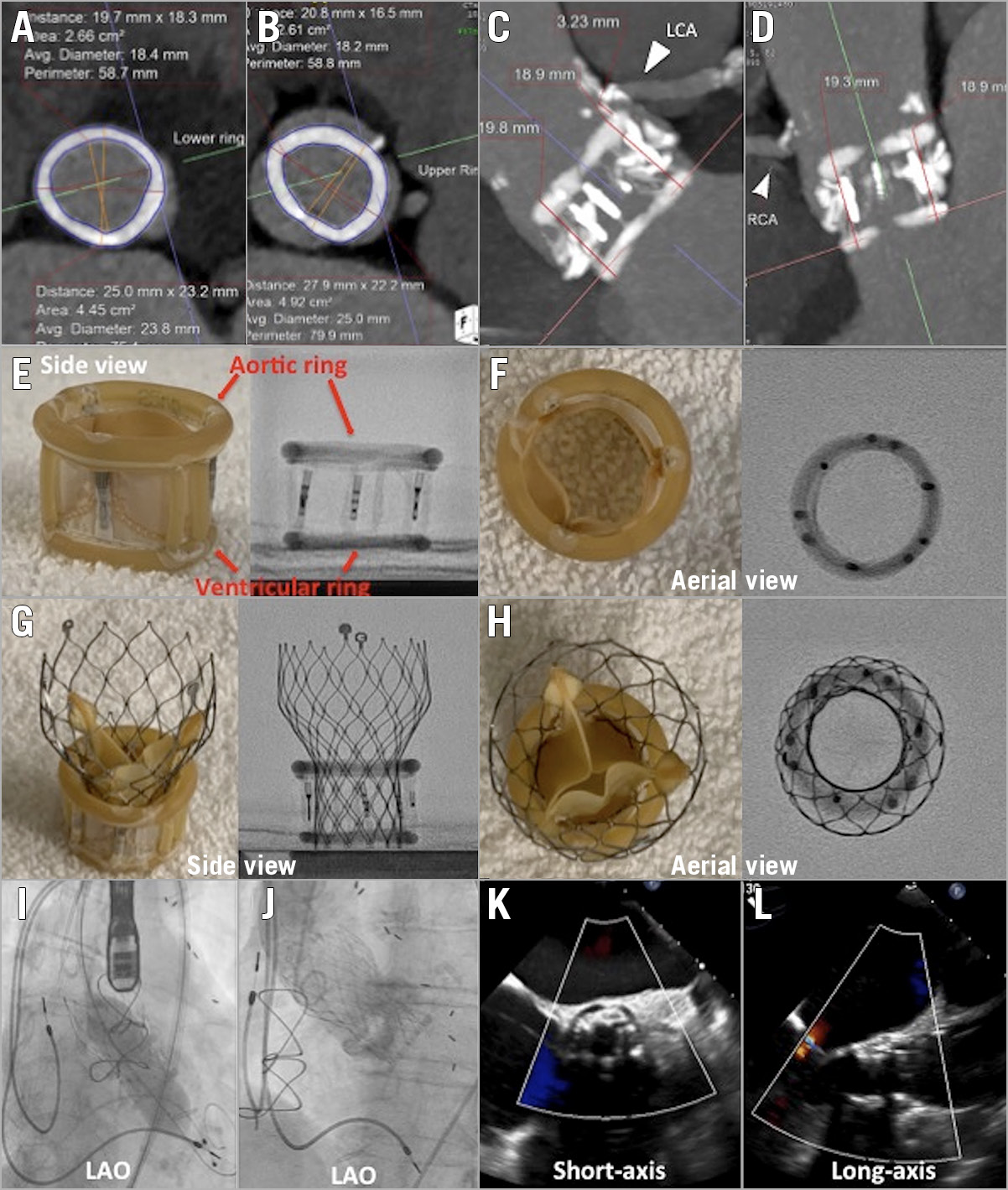
Figure 1. TAVI in Direct Flow Medical valve. Computed tomography (CT) dimensions of ventricular (A) and aortic rings (B). CT showing relationship between the Direct Flow Medical (DFM) valve and the left coronary (C) and right coronary (D) arteries. Benchtop side (E) and aerial (F) profiles of the DFM valve. Benchtop side (G) and aerial (H) profiles of the valve-in-valve. I) Post-dilatation of Evolut R valve. J) Final aortogram of valve-in-valve. TEE short-axis (K) and long-axis (L) view of valve-in-valve with colour Doppler.
Transcatheter aortic valve implantation (TAVI) has increasingly been performed in failing aortic bioprostheses, but these “valve-in-valve” procedures are technically more challenging1,2. The Direct Flow Medical® (DFM) transcatheter aortic valve (Direct Flow Medical Inc., Santa Rosa, CA, USA) was implanted worldwide3,4 before its eventual withdrawal from the market. We describe a case of repeat TAVI in a failing DFM valve and discuss the technical considerations involved (no data in the PCR “Valve-in-Valve” app: https://www.pcronline.com/PCR-Publications/PCR-mobile-apps/Valve-in-Valve-Aortic-app).
An 81-year-old female with a history of coronary bypass and pacemaker insertion underwent TAVI for severe aortic valve (AV) stenosis with a 25 mm DFM valve. After the index TAVI, her mean AV gradient improved from 47 mmHg to 18 mmHg. Six years later, she presented with New York Heart Association (NYHA) Class IV symptoms with a decrease in her previously normal ejection fraction (EF) to 35% and severe bioprosthetic aortic stenosis (mean AV gradient 40 mmHg). She was considered high-risk for surgery. Computed tomography (CT) was critical to aid in sizing as well as assessing the risk of coronary obstruction. The two rings of the DFM valve are filled with epoxy-based polymer and the final size may vary. In this case of a 25 mm DFM valve, the average inner diameters were 18.4 mm and 18.2 mm for the ventricular and aortic rings, respectively (Figure 1A, Figure 1B). The coronary ostia originated from above the aortic ring of the DFM valve; as such, the risk of coronary occlusion was assessed as being low (Figure 1C, Figure 1D). Due to the small inner diameter, we chose a supra-annular valve to provide better haemodynamics. Thus, a 23 mm CoreValve® Evolut™ R (Medtronic, Minneapolis, MN, USA) was chosen. To understand the ideal deployment of the valve, we obtained a 25 mm DFM valve and implanted a 23 mm Evolut R valve on the benchtop to study the optimal implantation depth (Figure 1E, Figure 1F). As seen from the benchtop models and our clinical case, the base of the Evolut should be positioned in line with the base of the ventricular ring of the DFM (Figure 1G, Figure 1H). Predilatation was performed with a 16 mm balloon and the repeat TAVI procedure was successfully performed under moderate rate control pacing to reduce motion. The Evolut R valve appeared underexpanded on fluoroscopy and there was an initial residual gradient of 15 mmHg. Therefore, post-dilatation was performed with a 20 mm TRUE® balloon (Bard Peripheral Vascular, Tempe, AZ, USA), with optimal results (Figure 1I-Figure 1L, Moving image 1-Moving image 4). At one-month follow-up, the patient had NYHA Class II symptoms with a mean valve gradient of 7 mmHg.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Post-dilatation of the Evolut R valve.
Moving image 2. Final aortogram of valve-in-valve.
Moving image 3. TEE short-axis view of valve-in-valve with colour Doppler.
Moving image 4. TEE long-axis view of valve-in-valve with colour Doppler.

