
Percutaneous mitral valve-in-valve (ViV) is an emergent option for patients with prosthetic dysfunction at high surgical risk1-3.
We present the case of a 69-year-old woman with heart failure and haemolytic anaemia due to severe mitral bioprosthesis insufficiency, with intraprosthetic and paraprosthetic regurgitation (one major leak point and two minor ones).
The patient had surgery to the mitral valve (MV) on four previous occasions – mechanical prosthetic MV replacement due to rheumatic mitral stenosis (1980), later replaced by a new mechanical prosthesis due to prosthesis dysfunction (1994), surgical closure of mitral paravalvular leak (2002), and finally, mechanical valve replacement (2008) by a nº27 bioprosthesis (Edwards Lifesciences, Irvine, CA, USA). Paravalvular leak closure (PLC) was attempted twice but failed, because device placement worsened valve dysfunction. The patient was at very high risk for cardiac surgery (EuroSCORE 15.3%) and thus transcatheter ViV implantation with simultaneous PLC was considered, using a transseptal approach. The maximum and mean transprosthetic gradients were 28 and 8 mmHg, respectively (prosthesis area was not calculated due to mitral and aortic regurgitation [AR]). The aortic valve was only mildly calcified; there was moderate AR, the aortic valve anatomic area was 1.6 cm2, but the mean aortic gradient was 44 mmHg (probably due to the hyperkinetic state).
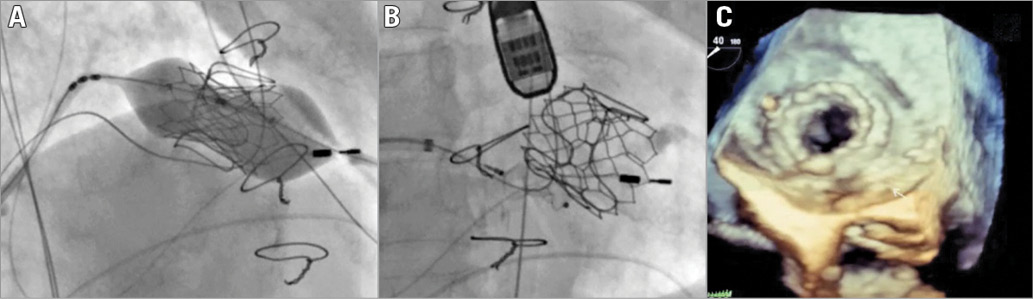
Puncture and dilatation of the interatrial septum were performed through the right femoral vein using two 8 mm balloons. Two guidewires were passed, one through the major leak and the other through the valve. They were entrapped in the left ventricle using a snare, creating two arteriovenous loops to allow traction when placing the valve and the leak closure device. The new 29 mm SAPIEN 3 bioprosthetic valve (Edwards Lifesciences) was delivered under rapid ventricular pacing (Panel A), followed by placement of a 10/3 mm AMPLATZER Vascular Plug III (St. Jude Medical, St. Paul, MN, USA) (Panel B, Moving image 1). Device placement did not cause prosthesis dysfunction. The final result was good, with improvement of intraprosthetic and paraprosthetic regurgitation (Panel C, Moving image 2). Final transprosthetic mitral maximum and mean gradients were 25 and 9 mmHg, respectively, within the range described after ViV4; final aortic mean gradient was 39 mmHg.
From a clinical standpoint the patient improved. Diuretics were successfully titrated to a fixed oral dose and blood transfusions were significantly less frequent.
Acknowledgements
This procedure was only possible with the help of Doctor Eulogio García plus Doctor Vasco Gama and co-workers.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Interatrial septal enlargement with two balloons, valve-in-valve implantation and paravalvular leak closure.
Moving image 2. Intraprosthetic and paraprosthetic mitral regurgitation before and after valve-in-valve implantation and paravalvular leak closure.
To read the full content of this article, please download the PDF.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Interatrial septal enlargement with two balloons, valve-in-valve implantation and paravalvular leak closure.
Moving image 2. Intraprosthetic and paraprosthetic mitral regurgitation before and after valve-in-valve implantation and paravalvular leak closure.

