Case summary
Background: A 49-year-old female presented with worsening exertional dyspnea (NYHA class III) and orthopnea for several months. Previous medical history was significant for rheumatic mitral regurgitation and three previous mitral valve replacements (MVRs).
Investigation: Transesophageal echocardiography (TEE) revealed severe mitral paravalvular leak (PVL) with two jets visualised. Cardiac computed tomography angiography (CTA) revealed a larger PVL and a smaller defect.
Diagnosis: Severe mitral paravalvular leak.
Treatment: Transcatheter mitral paravalvular closure.
Keywords: percutaneous, paravalvular leak, mitral regurgitation, prosthetic valve, transseptal puncture.
How should I treat?
Presentation of the case
A 49-year-old female presented with worsening exertional dyspnea (NYHA class III) and orthopnea for several months. Previous medical history was significant for rheumatic mitral regurgitation and three previous mitral valve replacements (MVRs). Transesophageal echocardiography (TEE) revealed severe mitral paravalvular leak (PVL) with two jets visualised. Laboratory investigations showed significant haemolytic anaemia (haemoglobin (Hb) between 8-9 g/dL) that required blood transfusion. Due to the excessive risk for a fourth operation, she was considered for percutaneous PVL closure.
Cardiac computed tomography angiography (CTA) revealed a larger PVL from the 2-4 o’clock position (view from top, anterior mitral annulus adjacent to the aorta being 12 o’clock), and a smaller defect at the 10 o’clock position (Figure 1). The plan was to close the larger defect first, and if subsequently still symptomatic, the smaller defect.
The procedure was performed under general anaesthesia with TEE and fluoroscopic guidance. Transseptal puncture was attempted using the standard technique. Due to the grossly thickened and calcified septum, the Brockenbrough needle could not pierce the septum, and during one attempt, the needle and the Mullins sheath slipped upwards and anteriorly, resulting in inadvertent aortic puncture (Figure 2a and b). Immediate cardiac surgical consult was obtained.
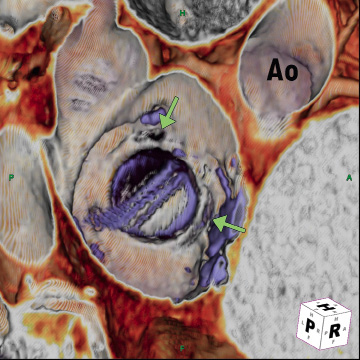
Figure 1. Cardiac CTA showing PVLs at the 10 o’clock and 2-4 o’clock positions (Aorta [Ao] is 12 o’clock).
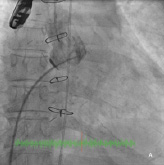
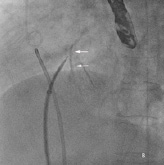
Figure 2. A) Fluoroscopy showing Mullins sheath in the aorta; B) Fluoroscopy showing Brockenbrough needle against thickened and calcified septum (arrows).
How could I treat?
The Invited Experts’ opinion
Redo surgery for mitral valve replacement is complicated by the repeat injury to the mitral annulus and has probably contributed to the recurrence of paravalvular leak (PVL) in this patient. Further surgical intervention in these patients can be associated with high operative mortality and morbidity1 giving rise to attempts of percutaneous transcatheter closures of PVL with variable procedural success. The major challenge of transcatheter mitral PVL closure lies in the ability to adequately visualise the area of interest to facilitate defect crossing and closure.2
Given the size and multiplicity of the PVL in this patient, we would have re-operated ab initio this patient with re-implantation of a new prosthesis. In this context of inadvertent aortic puncture, several perioperative precautions as cannulation of the femoral vessels and circulatory assistance prior to entry to the thorax can considerably reduce the risk of the procedure. The first step is repair of the aortic puncture with pledgeted sutures on both the aortic and atrial sides to avoid future atrio-aortic fistula. Thereafter, and in case of severe damage to the mitral annulus, intra-atrial insertion of the prosthesis can be performed after explantation of the prosthetic valve.
The new prosthesis is modified by enlarging the circumference of the sewing ring with a Dacron collar which is then sutured to the left atrial wall above the destroyed mitral annulus ensuring adequate fixation.3 If the fibrous tissue underneath the aortic valve is inadequate to secure another prosthetic valve without affecting the function of the aortic valve, a combined transatrial and transaortic approach can allow reconstruction of the mitral annulus underneath the aortic valve from fibrous trigone to fibrous trigone with a crescentic patch of Dacron or pericardium.4
Despite all the precautions taken during explantation of a previous valve, the mitral annulus may not support a new prosthetic valve. This is one of the reasons a redo operation is associated with a greater risk and reconstruction of the mitral annulus makes reoperative mitral valve surgery safer.
How could I treat?
The Invited Experts’ opinion
The accepted estimated complication rate surrounding transseptal procedures, even in skilful and experienced hands is approximately 1%1. In view of the particular anatomical orientation of the inter-atrial septum, these complications can have a dreadful course and potential to rapidly deteriorate into exitus of the patient. Directing the puncture needle too posterior will perforate the right atrial wall into the pericardial space; conversely aiming too anterior might perforate the aortic wall.
It is therefore of utmost importance that operators embarking on these kinds of procedures are aware of the risks and ready to react appropriately when needed. In approaching the case described above, several important issues should be taken into account.
First, the patient had already undergone three thoracotomies for successive mitral valve procedures. We agree that a fourth sternotomy would come with an additional risk2. However, according to the STS PROM risk score model, a fourth re-sternotomy, per se, would only modestly augment operative mortality risk (STS risk calculator see web page: http:// 209.220.160.181/STSWebRiskCalc261/). Therefore, a thorough patient risk assessment is essential, and a patient tailored decision should preferably be taken by heart team consensus. Second, after three previous mitral valve replacement procedures, the operator can anticipate more or less subtle changes in the typical anatomical interrelations (e.g., distortions, sutures) together with excessive scar tissue formation that might influence the transseptal puncture technique. We agree with the authors in performing the transseptal puncture using not only fluoroscopic, but also transoesophageal echocardiograhy (TOE) guidance. In our practice, we preferably use intracardiac echocardiography to guide the transseptal puncture.
The presented complication – with an iatrogenic puncture from the right atrium into the aorta landing both the Brockenbrough needle and the Mullins sheath in the aorta – demands immediate action by the operator. It is of paramount importance not to withdraw the Mullins sheath from the aorta, as it helps sealing off the puncture site and also secures valuable percutaneous treatment options. With the initial arguments favouring percutaneous approach over re-sternotomy in mind, an attempt to close the iatrogenic defect percutaneously would seem reasonable. As we work in hybrid suites equipped for both interventional and conventional surgical procedures, an experienced cardiothoracic surgeon should nevertheless be consulted so that within minutes a thoracotomy could be established when needed.
The Mullins sheath should first be advanced over the Brockenbrough needle well into the ascending aorta. The Brockenbrough needle can then be replaced by a 260 cm 0.035’’ Amplatz Super Stiff® guidewire to be advanced well into the aorta. An 8 Fr Mullins sheath has an outer diameter of 2.67 mm. In principle, the defect created by this sheath is therefore well defined and fairly small. The size of the created defect, and the relation to surrounding structures including the aortic valve, can be further appreciated by contrast injections through the Mullins sheath and by TOE. In view of the findings we would attempt to close the defect with the off-label use of an Amplatzer® Patent Foramen Ovale (PFO) or Duct occluder or comparable devices, depending on the immediate availability in the cathlab. The size of the Mullins sheath can accommodate most of these particular devices.
If successful, this poses an elegant catheter based solution of a potentially fatal complication of transseptal puncture into the aorta. It might be wise to stop there and extensively evaluate why the complication occurred, before embarking on further catheter based closure attempts of this initial paravalvular defect. A direct transapical approach could hereby present an interesting and more controlled alternative solution3.
How did I treat?
Actual treatment and management of the case
Due to the three previous cardiac surgeries, the consensus was that the risk of cardiac tamponade or exsanguination from removal of the Mullins sheath was remote. Therefore, after careful consideration, a joint decision was made to leave the Mullins sheath in situ and proceed with the transcatheter PVL closure, with cardiac surgical back-up in the catheterisation laboratory.
Repeated attempts at transseptal punctures were unsuccessful as the Brockenbrough needle could not penetrate the thickened and calcified septum (Figure 2b). A switch to the retrograde approach was made. Using a 5 Fr AR1 catheter, the Glide wire (Terumo, Tokyo, Japan) was advanced across the larger defect (Figure 3a). The catheter however, could not cross the defect despite positioning the Glide wire into the left lower pulmonary vein. The AR1 catheter was removed and a 4 Fr Glide® catheter was successfully advanced across the defect into the left lower pulmonary vein (Figure 3b). The Glide wire was replaced with a 0.035-inch extra-stiff Amplatz wire (Cook, Bloomington, IN, USA). A 6 Fr balloon-tipped catheter was then used to straighten the wire loop (to reduce likelihood of the Amplatz wire prolapsing back into the LV during sheath delivery) (Figure 4a, 4b, 4c). This facilitated advancement of a 7 Fr Shuttle sheath (Cook, Bloomington, IN, USA) from the femoral artery to the LA (Figure 5). A 10/8 mm Amplatzer® Ductal Occluder (ADO) was then deployed (Figure 6). TEE showed reduction in regurgitation through the medial PVL. The decision was made not to proceed with closure of the residual leaks but to observe the clinical course.
Although cardiac surgical opinion was that the Mullins sheath could be safely removed as haemodynamics remained stable, a step-wise approach to “test” the haemodyanmic stability of Mullins sheath removal was performed. A catheter was advanced to the right atrium (RA) from a separate femoral vein access. Monitoring both the blood pressure and RA pressure continuously, the 8 Fr Mullins sheath was removed over a 0.035-inch wire and rapidly replaced with the 4 Fr Glide catheter (Figure 7a). As haemodynamics remained stable, the 4 Fr Glide catheter was removed after 15 minutes leaving the 0.035-inch wire across the aortic puncture (Figure 7b). This wire was finally removed after 15 minutes of haemodynamic stability.
The patient experienced symptomatic improvement with resolution of her orthopnea. Hospital course was complicated by intracranial haemorrhage (despite a subtherapeutic level of oral anticoagulation) that required emergency craniectomy. She made a gradual recovery and was discharged 29 days post-procedure with no neurological deficit. At 2-month follow-up, she was in NYHA class II and had no orthopnea. There was a sustained rise in Hb to a baseline of 10 g/dL (without transfusion). TEE showed at least moderate paravalvular regurgitation from both defects, with residual leak still present from the larger defect. Repeat cardiac CTA showed a well expanded stable device and the residual defect at the 2-3 o’clock position (Figure 8).
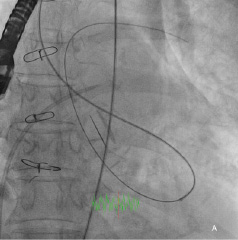
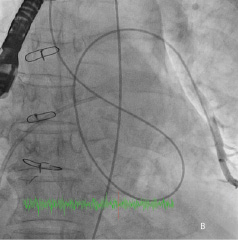
Figure 3. A) Fluoroscopy showing retrograde crossing of the Glide wire into the pulmonary vein; B) Contrast injection via the Glide catheter into the pulmonary vein.
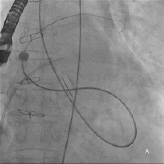
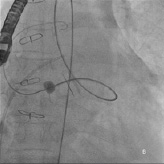
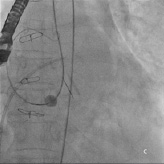
Figure 4. A, B, C) Fluoroscopy showing balloon-tip catheter anchored on the LA side straightening the 0.035-inch support wire loop.
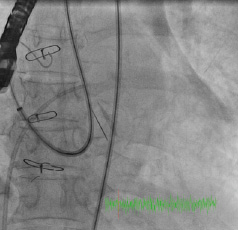
Figure 5. Retrograde advancement of the Shuttle sheath into the LA.
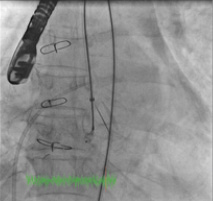
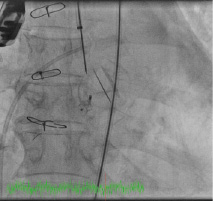
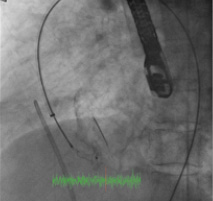
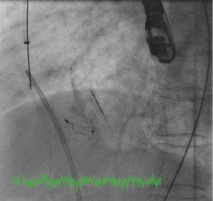
Figure 6. Deployment of the ADO device before (top) and after release (bottom).
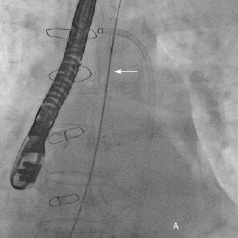
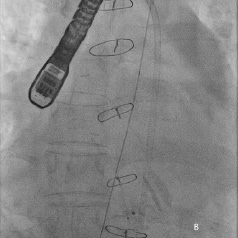
Figure 7. A) Mullins sheath in aorta removed over a 0.035-inch wire and replaced with a 4 Fr Glide catheter (arrow); B) 0.035-inch wire across the aorta after removal of the Glide catheter.
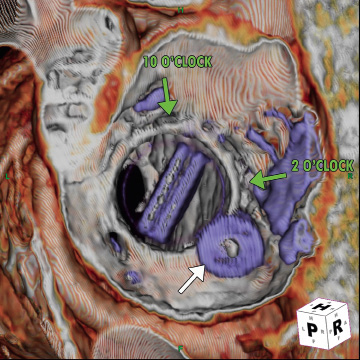
Figure 8. Cardiac CTA showing the ADO with residual defects.
Discussion
Most reports of percutaneous mitral PVL closure have employed the antegrade approach from a transseptal route with or without use of a snare; less commonly a retrograde approach has also been used although this invariably has been with a transseptal access to snare the wire for exteriorisation to form an arteriovenous loop1-6. With both approaches, the delivery sheath is advanced antegrade for device deployment as it is believed that this enhances the stability of the device, particularly for ADOs as the larger disk would be on the LV side7.
Our case has several learning points. Despite TEE and fluoroscopy, inadvertent aortic cannulation from transseptal puncture can still occur (in this case due to the needle and sheath slipping off a thickened and calcified septum). We describe a novel approach that allowed safe removal of the Mullins sheath from the aorta. The sheath was replaced with a smaller catheter to “test” haemodynamic stability with close monitoring of BP and more importantly RA pressure (as this is likely to rise first in cardiac tamponade). The smaller catheter served to keep the defect “plugged” while the wire would allow the Mullins sheath to be replaced quickly should haemodynamic decompensation occur. This case was unique however, as the risk of cardiac tamponade or exsanguination was low due to the three previous open MVRs; furthermore, the low position of the aortic cannulation made it anatomically likely that the puncture occurred directly from RA into the aorta (and not traversing the triangle of Koch) and therefore, no risk of tamponade or exsanguination.
We demonstrate that using an isolated retrograde approach to mitral PVL closure is possible. The issue of sufficient wire support to bring the delivery sheath into position was managed by placing the support wire deeply into the LA/ pulmonary vein, and by straightening the LV wire loop using a balloon-tipped catheter. This novel technique allowed the sheath a more “direct” route across the PVL and reduced the likelihood of the wire prolapsing out of the PVL. It also illustrates that stable retrograde deployment of ADO can be achieved, despite the theoretical concern that the regurgitant jet could dislodge the device7, with the caveat that a device large enough for the defect is selected. Lastly, in patients with multiple PVLs, symptomatic improvement can be achieved by partial closure.
Conclusion
Our case illustrates a transseptal complication of percutaneous mitral PVL closure and the difficulties encountered during the retrograde closure approach which were overcome by novel transcatheter techniques. This case also demonstrates that retrograde deployment of the ADO can be stable despite the theoretical concern of embolisation if appropriate device size had been selected. Finally, a valuable learning point is that symptomatic relief can be achieved with partial PVL closure, and attempts to completely close all leaks may not be necessary.

