Abstract
The incidence of paravalvular leaks after surgical valve replacement is estimated to be 2-17%. Paravalvular leaks (PVL) can be asymptomatic and not require treatment or can cause haemolysis or heart failure. If symptomatic or if the severity of the leak is moderate or severe, redo surgery is a therapeutic option, but this is accompanied by a high perioperative risk and a high recurrence rate. A lower risk alternative is percutaneous PVL closure, with a 1-2% risk of periprocedural death or need for reoperation. These procedures are often intricate, which is reflected by a rather modest rate of procedural success (reported to be around 80%). This requires that better technical solutions become available in the future. Today, only two dedicated devices for PVL closure exist, the AMPLATZER Vascular Plug III and the paravalvular leak device. Besides, many non-dedicated devices are used, such as atrial septal occluders, ventricular septal occluders and a variety of vascular plugs. While aortic PVL are approached with a retrograde transarterial approach, mitral PVL can be approached using either an antegrade transvenous approach (transseptal), a retrograde transapical approach or, rarely, a retrograde transaortic approach.
The challenge of paravalvular leak treatment: why surgery cannot be the optimal therapy - the need for less invasive options
Paravalvular leak (PVL) is a well-known complication after surgical valve replacement, with a reported incidence at follow-up of 2% to 17%, both in the mitral and in the aortic position1-3. Among patients in whom PVL develops, approximately 3% require treatment because of heart failure, haemolysis, or a combination of both4,5.
In symptomatic patients, surgical closure of a PVL or valve re-replacement is associated with improved event-free survival compared to conservative management3. Although surgery remains the most common therapy for these defects, re-operative surgery is associated with relevant morbidity and mortality, as well as a high risk of recurrence1,3,6 (Figure 1). We recently reported the longest follow-up (up to 14 years) of a large series of patients who underwent surgical treatment of PVL in a tertiary high-volume surgical centre7: perioperative mortality was more than 10% and survival at follow-up was less than 40% at 12 years. Considering the mean age of the study population was less than 62 years, this underlines the need for alternative, safer options. Mortality risk was increased in patients suffering from chronic renal failure and in patients who had more than one previous open heart surgery. Only three patients underwent a second reoperation during follow-up due to recurrent leak. This does not, however, reflect the real incidence of PVL recurrence after surgical treatment of PVL, since the majority of patients with recurrent PVL are deemed too high risk for a second redo surgery and are therefore treated conservatively. It has been shown that surgical mortality increases with the number of reoperations: 13% after the first, 15% after the second, and 37% after the third redo surgery1,2,4.
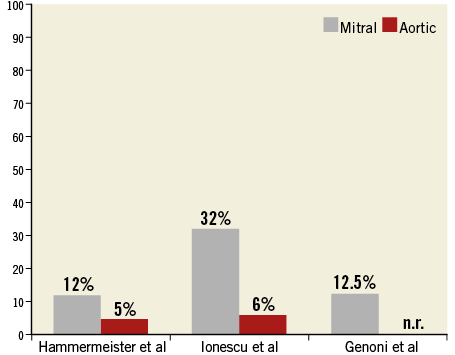
Figure 1. Recurrence rates after surgical treatment of PVL are higher in mitral than in aortic position. n.r.: not reported
Other series have reported in-hospital mortality between 6% and 22% after surgical reoperation for PVL, with reported risk factors for mortality being severe symptoms (New York Heart Association [NYHA] Class III-IV, severe haemolysis)3,5, and mitral PVL7.
The suboptimal results from surgical repair of PVL suggest that a less invasive solution should be considered the first-line therapy in these high-risk patients.
Percutaneous PVL closure, which was first described by Hourihan et al in 1992, has emerged as an alternative to surgical closure and has been found effective if successful8.
Catheter-based treatment of PVL: lucky bags from simple to intricate!
Transcatheter closure of PVL represents one of the most intricate procedures in the area of structural heart interventions, largely depending on the location (the most difficult site being the medial border of the mitral valve) and size of the defect, its anatomy, and the experience of the operator. Moreover, not uncommonly multiple defects are present. Therefore, the reported success rate of PVL closure varies from 60% to 90% in different series9-13.
Endpoint definitions for percutaneous PVL closure are not well defined, but are often divided into: 1) technical, 2) procedural, and 3) clinical success. Technical success is defined as stable device position within the PVL, and lack of new prosthetic valve dysfunction. Procedural success is defined as technical success and PVL reduction. Clinical success is defined as symptom relief and has been shown to be related to the degree of residual regurgitation. Furthermore, clinical success is greater for patients with symptoms of heart failure than for those with haemolysis. Sorajja et al9 reported short-term outcomes with closure of 141 defects in 115 patients (78% mitral PVL, 22% aortic PVL), with a procedural success of 77% (76% with mitral PVL vs. 80% with aortic PVL). Only 10% of patients were left with regurgitation more than moderate-to-severe; 8.7% of patients experienced a major adverse event at 30 days (sudden death, 1.7%; stroke, 2.6%; emergency surgery, 0.9%; bleeding, 5.2%). Two devices embolised during the procedure, but were retrieved without sequelae. One patient required emergent surgery because of valve interference of a device that could not be retrieved percutaneously, and there were no procedural deaths. The same group reported a three-year survival of 64%, which was unrelated to the degree of residual regurgitation; 72% of patients who survived had minimal or no exertional dyspnoea at three years. Of note, NYHA functional class improved only in patients with residual regurgitation being mild or less14. Early data for percutaneous PVL repair suggested similar survival compared with reports in the surgical literature, with worse outcomes noted in patients treated with conservative medical management. Patients with haemolysis require a greater degree of closure, which can be technically challenging.
The risk for emergent surgery and for death as a complication of percutaneous PVL repair is 1%-2%10,15. The most common complication associated with percutaneous PVL repair is bleeding, which may occur at the access site or due to pericardial effusion. Another possible complication is interference of the device with the prosthetic leaflets in the case of a mechanical valve. In case of embolisation of the device, snaring and retrieval are usually successfully performed, while surgery is rarely needed.
Technical aspects
DEVICE CHOICE
The ideal device for PVL closure should be retrievable and repositionable, show good conformability (in order to adapt itself to the typically semilunar and irregular three-dimensional structure of the defect), have a low-profile deliverability and result in complete sealing after implantation.
Current devices used for PVL closure are all retrievable and repositionable. They consist of a woven nitinol mesh and some are filled with polytetrafluoroethylene or polyethylene terephthalate.
The only devices specifically dedicated to PVL occlusion are the crescent-shaped AMPLATZER Vascular Plug III (AVP III; St. Jude Medical, St. Paul, MN, USA)16 and its derivative, the Occlutech paravalvular leak device (PLD) (Occlutech, Helsingborg, Sweden)17.
The AVP III is a nitinol-based device with an elliptical lobe and a disc on each side. The configuration of this device allows it to adapt to the often crescent-shaped defects. Different sizes from 4×2 mm to 14×5 mm are available, fitting through a 4-7 Fr sheath. The two discs on each side extend the central lobe by 2 mm, thereby reducing the risk of interference with mechanical prosthetic valve leaflets13. Nietlispach et al first reported feasibility, safety, and efficacy of AVP III for PVL occlusion in both the mitral and the aortic position in a small series of high-risk patients with severe paravalvular regurgitation13. Although a direct comparison between different devices has never been performed, this initial experience was important since it suggested that the use of dedicated devices for PVL occlusion was crucial to achieve procedural and clinical success. Cruz-Gonzalez et al reported a series of 33 patients with 34 PVLs (27 mitral, seven aortic) undergoing transfemoral PVL closure using the AVP III18. The device was successfully implanted in 94% of patients and successful closure (defined as regurgitation reduction ≥1 grade) was achieved in 91% of patients. At 90 days, survival was 100%, and more than 90% of patients had significant clinical improvement.
The PLD is a double-disc device which comes in either a square or a rectangular shape, one disc being slightly larger than the other. The two discs are connected by a round or elliptical waist or a small connector. The sizes of the larger disc vary from 11.5 to 21 mm and require a 6-10 Fr sheath. Early results reported with the novel Occlutech device showed a 100% procedural success in 21 treated patients, with no procedural mortality17.
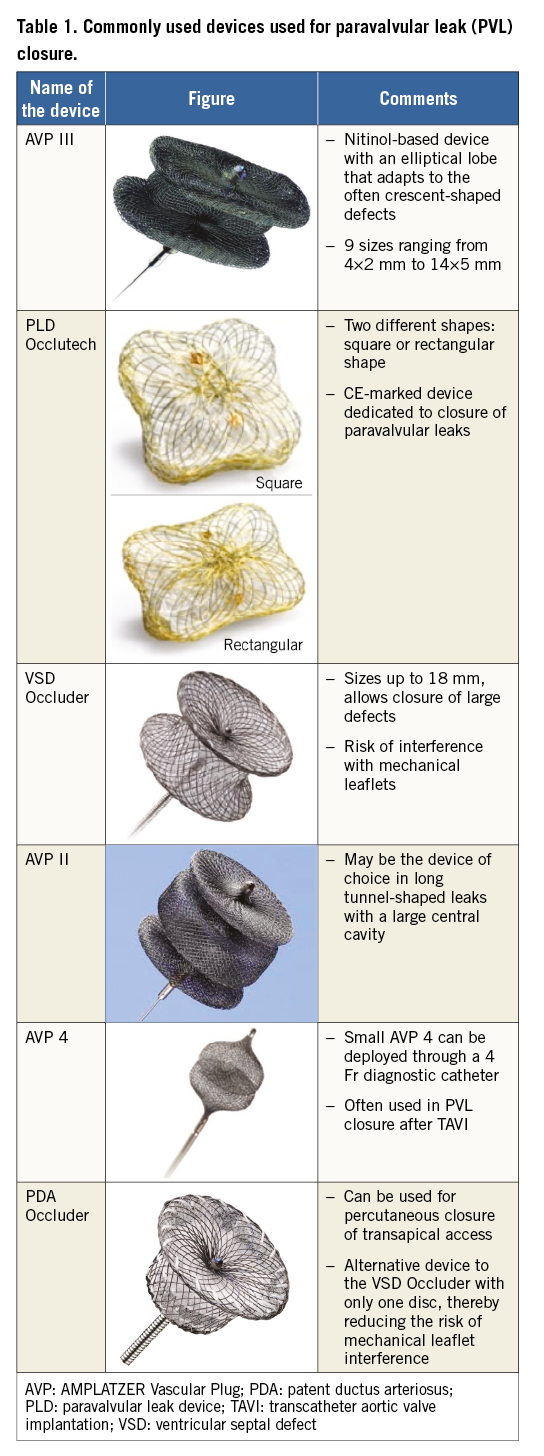
According to the anatomy and location of the defects, different AMPLATZER or “AMPLATZER-type” devices may be used for PVL closure, such as ventricular septal defect occluders, Vascular Plug II and 4, and duct occluders. Table 1 lists the different devices and their specific features and indications.
In case of large defects, sequential devices can be used.
Choice of access
MITRAL PARAVALVULAR LEAKS
Mitral percutaneous PVL repair can be performed using the antegrade (venous, transseptal), retrograde (arterial), or transapical (TA) access. Access-site selection depends on the location of the prosthesis, the location of the defect in relation to the valve, the presence of mechanical valves, operator experience and preference, and the anatomical peculiarities of the individual patient. The procedure is typically performed under general anaesthesia using transoesophageal echocardiographic (TEE) and fluoroscopic guidance.
Our first-line approach is antegrade transseptal, even for medial leaks. The main advantage of this access is its minimal invasiveness. The disadvantages can be difficulties in wiring the defect (due to a lack of coaxiality) and difficulties in crossing the defect with the delivery sheath despite the use of stiff guidewires. A lower rather than higher transseptal puncture is desirable. The use of a steerable sheath, such as the Agilis™ sheath (St. Jude Medical, St. Paul, MN, USA), allows navigating the sheath in front of the defect and facilitates wire passage (Figure 2, Figure 3). Usually a 0.035” hydrophilic wire is used to cross the PVL (e.g., GLIDEWIRE®; Terumo Medical Corp., Shibuya, Tokyo, Japan). The GLIDEWIRE is then exchanged for a sturdy support wire (e.g., the Back-up Meier™ guidewire; Boston Scientific, Marlborough, MA, USA). The delivery sheath is then advanced through the defect over the support wire.
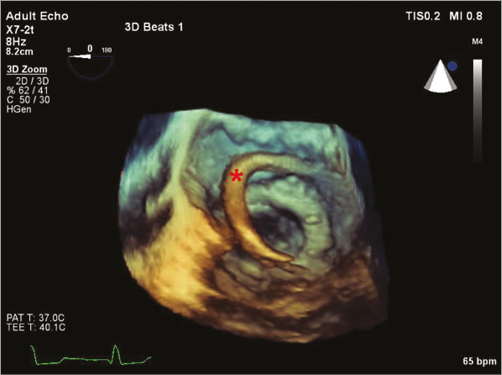
Figure 2. Three-dimensional transoesophageal echocardiographic view of the steerable Agilis sheath (marked with a red star) during a transseptal mitral posteromedial paravalvular leak occlusion.
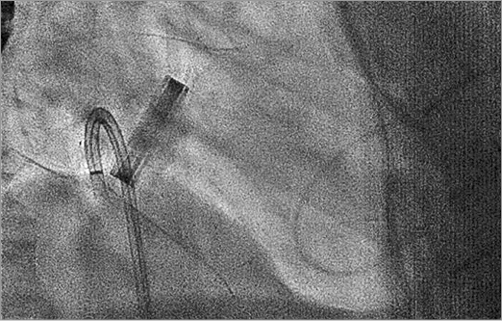
Figure 3. Wiring of a posteromedial mitral leak using the Agilis sheath, fluoroscopic right anterior oblique projection.
If the angle between the transseptal puncture and the defect is unfavourable (e.g., medial leaks adjacent to the interatrial septum), crossing the defect with the delivery sheath can be impossible. To overcome the lack of support to cross the defect, the support wire can be exteriorised either by creating a veno-arterial loop (snaring the wire via the transfemoral arterial access), or by exteriorising the wire through the cardiac apex12. While the latter further improves coaxiality, it considerably increases the invasiveness of the procedure.
Once the delivery sheath is across the defect, the ventricular part of the device is deployed. The atrial part is then deployed after retracting the sheath. The efficacy of the implant is controlled by TEE. A “push-pull test” is performed and free movement of the prosthetic valve leaflets (in case of a mechanical prosthesis) is confirmed before final device deployment. If needed, more than one device can be implanted sequentially (Figure 4).
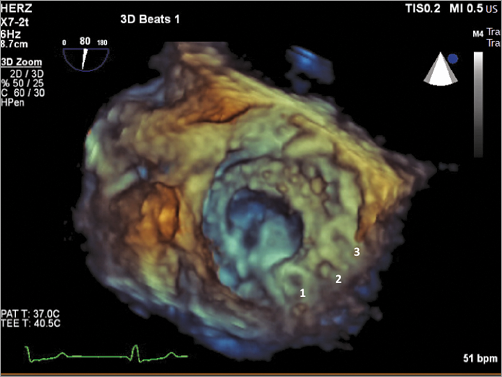
Figure 4. Surgical three-dimensional transoesophageal view of multiple occluders (n=3) implanted sequentially to occlude a medial leak.
TA access is a safe alternative for mitral transcatheter PVL closure. The potential advantage is the often less difficult wiring of the defect (in the direction of the jet) and encountering less resistance to cross the defect with the delivery sheath. We recently reported satisfactory acute results of a small series of 17 very high-risk patients who underwent mitral PVL closure through the transapical route. Notably, 30-day mortality was 0%, with an acute procedural success of 94%, and these results compared favourably with open heart surgery19. In another series consisting of 43 patients by Ruiz et al where the TA access was used for the majority of mitral PVLs, technical success rate for device deployment in mitral PVLs was 89%, although the precise percentage of TA cases was not reported12. TA access can be achieved either by a surgical cut-down through a small anterolateral left thoracotomy, or by direct percutaneous puncture of the left apex. In the latter case, there is the potential risk of accidentally puncturing the left anterior descending coronary artery. A selective coronary angiogram can be performed to guide the puncture. At the end of the procedure, the sheath is removed from the muscular apex with self-sealing of the myocardium (in case of small sheaths) or percutaneous closure of the puncture site by the implantation of an AMPLATZER Occluder device (usually VSD or a duct occluder)20 (Figure 5).
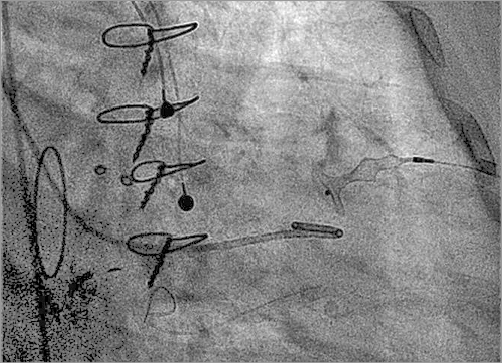
Figure 5. Percutaneous closure of the apical puncture site by the implantation of a ventricular septal defect occluder.
At the moment, in the absence of direct comparisons of the different approaches, clear evidence is lacking as to what the optimal access for mitral PVL is. Although we firmly think that the decision on the best approach should always be patient-tailored, a transseptal approach should be, in our opinion, the first-line option whenever feasible, since it represents the least invasive method. On the other hand, it has been shown that the transapical access, providing a more direct approach, leads to a significant decrease in procedure and fluoroscopy times21. In some centres, the retrograde arterial approach has almost been abandoned.
AORTIC PARAVALVULAR LEAKS
Closure of aortic PVL is technically less demanding and is mostly performed retrogradely from the femoral artery. Depending on the leak and the operator preference, the procedure can be performed under local anaesthesia with fluoroscopic guidance alone. The leak is again crossed with a hydrophilic 0.0035’’ wire using an Amplatz left 1 or a multipurpose catheter. Once the wire is in the LV, it is exchanged for a stiff support wire. Thereafter, the chosen device is deployed with the same technique described for the mitral location.
The importance of multimodality imaging
Pre-procedural planning typically includes TEE in all patients. Some operators advocate angio-computer tomography scans to get a better understanding of the three-dimensional structure of the leak and precise delineation of the tortuosity of the leak channel and of the extension of the leak on the annular plane.
Intraprocedural imaging consists of TEE (for most mitral and selected aortic PVL closure procedures). The use of three-dimensional TEE may help to improve procedural success. A common language between the echocardiographer and the operator is therefore important. An aortic PVL is described according to the vicinity to the coronary cusps (right, left, or non-coronary). Mitral PVLs are described either as medial (close to the interatrial septum), lateral, anterior (towards the aortic valve), posterior or using a clock scale with 12 o’clock being anterior.
Conclusions
Percutaneous treatment of PVL has significantly less morbidity than redo surgery and should represent the first-line therapy for most patients with symptomatic PVL. Transcatheter PVL closure, particularly of the mitral valve, is one of the more demanding procedures from a technical standpoint in the field of structural heart interventions, and procedural success depends on patient factors, anatomical factors, and on the volume and experience of the team performing the procedure. PVL closure often requires good imaging and a team approach. Dedicated devices and larger case series are needed to develop these procedures further and further improve outcomes.
Conflict of interest statement
F. Maisano, B. Meier and F. Nietlispach are consultants for St. Jude Medical. B. Meier received grants to the institution from St. Jude Medical. The other authors have no conflicts of interest to declare.

