
Abstract
Aims: The aim of the study was to assess the safety and efficacy of percutaneous closure of paravalvular prosthetic leak (PVL) and to identify the predictors of procedural success and early complications.
Methods and results: A total of 514 first-attempt percutaneous PVL closure in 469 patients were included at 19 centres. Technical and procedural success was achieved in 86.6% and 73.2% of the patients, respectively. In multivariate analysis, the independent predictors for procedural success in mitral lesions were the type of device used (AMPLATZER AVP III vs. others, HR 2.68 [1.29-5.54], p=0.008) and the number of procedures performed at the centre (top quartile vs. others, HR 1.93 [1.051-3.53], p=0.03). For aortic leaks the only predictor of procedural success was the leak size (≥10 mm vs. <10 mm, HR 3.077 [1.13-8.33], p=0.027). The overall major adverse events rate (death or emergency surgery or stroke) at 30 days was 5.6%; the only predictor for combined adverse events was New York Heart Association functional Class IV (HR 4.2 [1.42-12.34], p=0.009).
Conclusions: Percutaneous closure of PVL can be performed with a reasonable rate of procedural success and a low rate of major complications. The type of device used, the accumulated experience and the leak size are predictors of procedural success.
Abbreviations
AVP: AMPLATZER Vascular Plug
PVL: paravalvular leak
TEE: transoesophageal echocardiography
Introduction
Despite the advances in valve replacement techniques, periprosthetic regurgitation or paravalvular leak (PVL) remains a severe problem. Although most PVLs remain clinically silent, about 1-5% of leaks progress to severe regurgitation, develop clinical symptoms (haemolysis, heart failure or both1) and increase mortality2,3.
Until recently, surgery has been the only available therapy for the treatment of clinically significant PVLs. However, re-do surgeries are associated with a high rate of PVL recurrence and with a higher mortality rate than the index procedure3-6. Therefore, in the last few years percutaneous PVL closure has emerged as an alternative to surgical reoperation7,8. So far, the global experience remains limited to single-centre experiences9-11 and a recently published large series12.
There is a paucity of data on the feasibility and outcome, and predictors for success and complications of this promising procedure in multicentre studies. This study sought to assess the safety and efficacy of percutaneous closure of PVL and to identify the predictors of procedural success and early complications in a multicentre “real-world” registry.
Methods
This was an observational retrospective study. All patients in whom first-attempt percutaneous PVL closure was performed from November 2002 to January 2014 at 19 hospitals in Spain were included (SpanisH real-wOrld paravalvular LEaks closure [HOLE] registry). All patients signed informed consent and the institutional review board approved the study.
Patients with the following clinical criteria were considered for percutaneous repair: symptomatic heart failure or clinically significant symptomatic haemolytic anaemia (haemoglobin <13 g/dl in women or <15 g/dl in men, requiring transfusion, with laboratory evidence of intravascular haemolysis); moderately severe or severe paravalvular prosthetic regurgitation; and absence of active endocarditis. Patients were advised of the procedural risks and options, including open surgical correction.
The collected variables included: demographics, baseline characteristics, clinical indications for PVL closure, procedural characteristics including technical and procedural success, and periprocedural adverse events. Details regarding technical details of PVL closure have been published elsewhere1,7,13,14. An example of PVL closure is provided in Moving image 1-Moving image 10.
Definitions
Periprosthetic PVL was defined as a regurgitant jet, demonstrated by Doppler echocardiography, originating between the outer margin of the prosthetic sewing ring and the native tissues around the valve. Technical success was defined as successful delivery of a PVL closure device without interference with the valve prosthesis7. Procedural success was defined as technical success and ≥1 grade regurgitation reduction7. Finally, periprocedural adverse events (occurring within 30 days of the procedure) included all-cause death, myocardial infarction, complete atrioventricular block, stroke, air embolism, device embolisation, prosthetic leaflet impingement, emergency cardiac surgery, significant pericardial effusion or cardiac tamponade and vascular complications.
Statistical analysis
Continuous variables are presented as means (±SD) and categorical variables as frequencies and percentages. In case of skewed distribution, variables are shown as medians (interquartile range). Chi-square analysis was used to compare categorical variables.
Clinical and procedural parameters were tested for an association with major adverse events (death, stroke, and emergency surgery) and with procedural success. Those factors with p<0.1 on univariable analysis were entered into a multivariable stepwise regression model. Variables initially tested were: age; male sex; presenting symptoms (heart failure vs. haemolytic anaemia); prosthesis type (mechanical or biologic); history of coronary artery disease, chronic renal failure, pulmonary hypertension; leak location (anterior, septal, lateral or posterior for mitral leaks); leak size (maximum diameter ≥10 mm vs. <10 mm); logistic EuroSCORE; access technique (transapical or transfemoral); type of device (AMPLATZER™ Vascular Plug III [AVP III] [St. Jude Medical, St. Paul, MN, USA] vs. others); centre volume– number of procedures performed at the centre (top quartile vs. others); time from last surgery; NYHA functional class (IV vs. others). P-values ≤0.05 were considered statistically significant; a 95% confidence interval was used. All statistical analyses were performed with SPSS software, Version 20 (IBM Corp., Armonk, NY, USA).
Results
BASELINE PATIENT CHARACTERISTICS
A total of 514 first-attempt PVL closure in 469 patients were included in the database. Baseline patient characteristics are summarised in Table 1. All the procedures were to treat surgical valves. The mean age was 68.15±10.17 years. Heart failure was the predominant clinical indication for the procedure (38.9%), with haemolytic anaemia being present in 9.3% and both conditions in 51%. The mean logistic EuroSCORE was 17.52±11.56%. The mean time since last valve replacement to percutaneous closure attempt was 8.53±7.82 years.

PROCEDURE
Treated defects were most commonly paramitral (70.2%) and involved mechanical prostheses (88.7% mitral prostheses, 78.1% aortic prostheses). Transfemoral access was used in most of the patients (94.2%) and the antegrade transseptal approach was used in 44.5% of the patients (Table 1, Table 2, Figure 1). The most commonly used device was the AMPLATZER AVP III (Table 2, Figure 2). In 15% of the cases multiple devices were deployed and placed in single defects to achieve successful reduction in regurgitation (Figure 3).
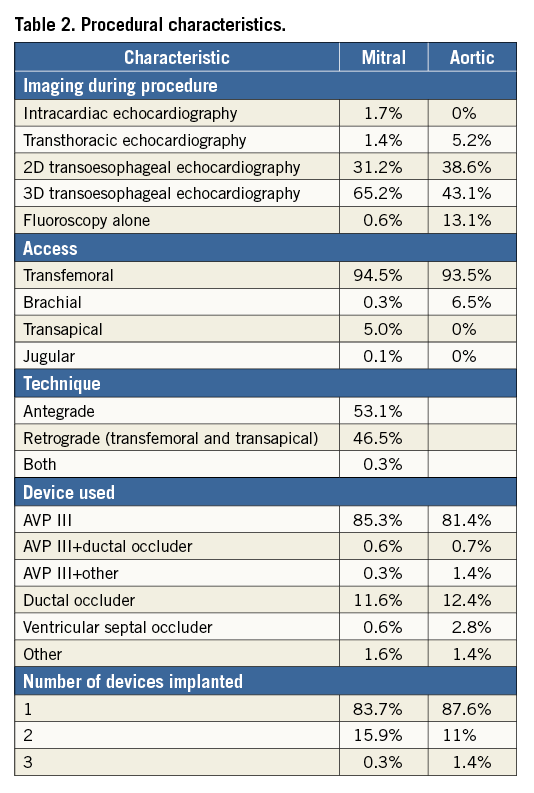
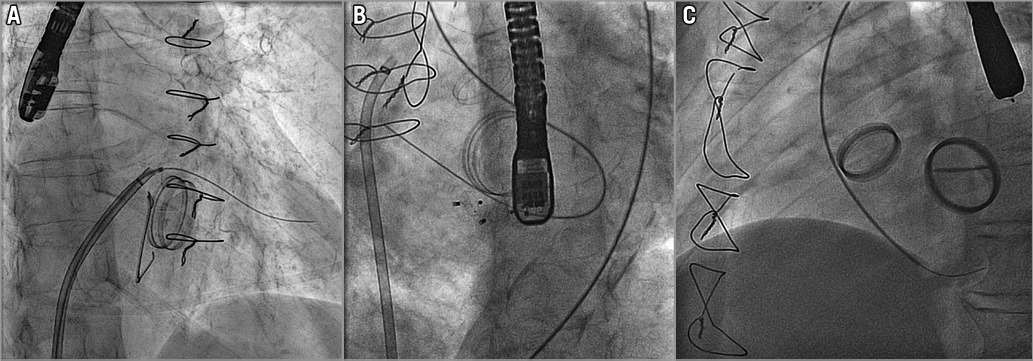
Figure 1. Paravalvular leak closure approaches. A) Antegrade transseptal approach for mitral paravalvular leak closure. B) Retrograde transfemoral approach for mitral paravalvular leak. C) Retrograde transfemoral approach for aortic paravalvular leak closure.
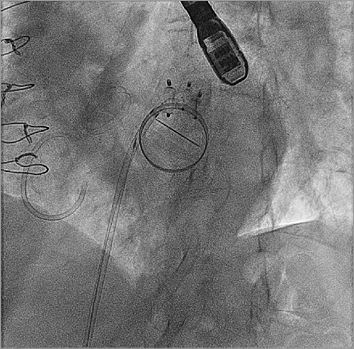
Figure 2. Fluorocospic image showing multiple devices placed in a single defect.

Figure 3. AVP III device images. A) 3D TEE image showing an AVP III device (arrow) placed in a paravalvular leak. B) AVP III device.
Global technical success was achieved in 86.6% of the patients (Table 2). Overall, procedural success occurred in 73.2% of the patients. The rates of technical success were slightly lower for perimitral lesions versus aortic lesions (84.8% vs. 90.8%, p=0.064). Similarly, the rates of acute procedural success were lower for mitral leaks vs. aortic leaks (70.6% vs. 74.2%, p=0.393), although these differences were not statistically significant.
In multivariate analysis, the independent predictors for procedural success in mitral lesions were the type of device used (AVP III vs. others, 85.7% vs. 68.2%, OR 2.68 [1.29-5.54], p=0.008) and the number of procedures performed at the centre (top quartile vs. others, 82.2% vs. 65.3%, OR 1.93 [1.051-3.53], p=0.03). For aortic leaks, the only predictor of procedural success was the leak size (maximum diameter ≥10 mm vs. <10 mm, 81.2% vs. 62.5%, OR 3.077 [1.13-8.33], p=0.027).
PERIPROCEDURAL ADVERSE EVENTS
There were no complications in 80.2% of the patients (Table 3). The most frequent adverse event was vascular complications and minor bleeding (8.6%). No major bleeding was reported. In six cases (3.5%) device embolisation occurred, but all of them were successfully snared and retrieved. Pericardial effusion was detected in 0.8% of the patients. The overall major adverse events rate (all-cause death, stroke, and emergency surgery) at 30 days was 5.6%. In multivariate analysis, the only independent predictor for combined major adverse events was NYHA functional Class IV (HR 4.2 [1.42-12.34], p=0.009). Similarly, the only predictor of all-cause mortality at 30 days was NYHA functional Class IV (HR 6.32 [1.94-20.8], p=0.002).

Discussion
The principal findings of this multicentre registry are the following: percutaneous repair of symptomatic PVLs can be performed with a relatively high rate of procedural success; these procedures can be performed in high-risk patients with an acceptable incidence of complications; several independent predictors of procedural success and early complications were identified. To our knowledge, this analysis represents the largest series of percutaneous PVL closures yet reported.
Percutaneous closure of periprosthetic PVLs has emerged as an attractive alternative to cardiac surgery, with lower morbidity and mortality rates in comparison to surgical series11,15. However, the success and complication rates vary between different series16, as this is a complex and technically demanding procedure. Different devices and techniques have been proposed for this procedure1, and there is evidence of a learning curve having occurred with the adoption of dedicated techniques for catheter delivery and echocardiographic imaging9. Furthermore, clinical experience thus far has been limited9-11,13,14,17,18. Two large case series10,11 in experienced centres with 57 and 141 PVL closures reported a technical success that ranged from 77-86%, and clinical success from 67-77%. Recently, the combined experience from the United Kingdom and Ireland has been published: 308 PVL closure procedures were attempted in 259 patients in 20 centres. Devices were successfully implanted in 91% of patients; PVL improved post procedure (p<0.001) and was none (33.3%), mild (41.4%), moderate (18.6%) or severe (6.7%) at last follow-up. Finally, a recent meta-analysis16 including 12 clinical studies with 362 patients showed that procedural success was achieved in 76.5% of cases, ranging from 29.6% to 100%. Technical success was achieved in 86.5% of cases, ranging from 62.5% to 100%. Successful transcatheter PVL reduction was associated with a lower cardiac mortality rate and with a superior improvement in functional class or haemolytic anaemia, compared with a failed intervention. Also, fewer repeat surgeries were observed after successful procedures.
The present investigation included 514 PVLs treated in 19 centres. This registry confirmed the results of previous studies10-12,17 by showing that device delivery was successful in 86.6% and successful percutaneous closure was achieved in 73.2% of patients. Remarkably, these results were achieved with relatively low rates of complications in a population at high risk for surgery. It should be noted that, as many of the cases have been performed in the last few years, 3D TEE and specifically designed devices were used in most of the cases. The adoption of specifically designed devices, dedicated techniques for catheter delivery and advanced echocardiographic imaging could partially explain these results.
PROCEDURAL SUCCESS PREDICTORS
Predictors of procedural success have not been described previously. We identified the type of device used (AVP III vs. others) and the number of procedures performed at the centre (top quartile vs. others) as independent predictors for procedural success in mitral leaks and the leak size in aortic leaks.
DEVICE USED
A number of devices not specifically designed for this procedure have been used to treat PVLs (i.e., AMPLATZER™ ASD Occluder device, AMPLATZER™ PDA Occluder, AMPLATZER Vascular Plug II or the AMPLATZER™ Muscular VSD Occluder; St. Jude Medical). However, most of these devices present several potential limitations. The AMPLATZER ASD Occluder device has a large distance between the waist and the discs (12-14 mm in most devices), which may increase interference with the discs of the prostheses; the first-generation AMPLATZER PDA Occluder had only one retention skirt, which could increase the risk of embolisation; the Vascular Plug II and the VSD Occluder have a round shape, and thus might not be suitable for non-round shapes.
The AVP III has an oval shape, multiple layers, more and thinner wires, smaller pore size, improved surface contact and faster occlusion compared to other AMPLATZER devices. Due to these characteristics and design, the AVP III is potentially an ideal device for this procedure and it has recently been used off-label for PVL closure, mainly in Europe.
Although there are few data on the AVP III, the results are promising; Nietlispach et al19, who described the initial experience with this device, obtained technical success in 100% of the five patients in whom it was implanted. Cruz-Gonzalez et al13 reported 90.9% success in 33 patients, Smolka et al20 reported 93.9% success in 46 patients, and Ozkan et al21 100% success in three patients.
Recently, Occlutech (Jena, Germany) obtained CE approval for its device specifically designed for PVL closure (Occlutech PLD). Goktekin et al reported the initial results on “first-in-man” use in 201422. In the present study, this device was used only in one patient; therefore, further work is needed to assess the safety and efficacy of this device, especially compared to the AVP III.
Therefore, device choice depends on the shape of the defect, the type of prosthetic valve (mechanical or biological), the access and whether it is planned to use a single device or multiple devices. For a small crescent-shaped leak, an AVP III or an Occlutech PLD is used most of the time. However, a large crescent-shaped leak can be treated either with one large device (e.g., a VSD Occluder), or with multiple purpose-specific devices. In case of a round PVL, a Vascular Plug II or the VSD Occluder would be the device of choice. If the leak is long tunnel-shaped, the AVP II could be used.
CENTRE VOLUME
It is well recognised that percutaneous repair of PVL is a challenging procedure. It has been previously reported that there is evidence of a learning curve in this procedure9. However, a comparison between more experienced versus less experienced centres has not previously been reported. In the present study, we showed a higher procedural success rate for mitral leaks in experienced centres. Recent expert consensus documents have highlighted the need for professional training in structural heart disease interventions due to the rapid growth in this field23; yet, there are virtually no data on the professional experience required to optimise clinical outcomes. The present data have implications for physician training and performance in complex structural heart disease interventions.
LEAK SIZE
A maximum leak diameter ≥10 mm has been identified as an independent predictor for procedural success in aortic leaks. Devices rarely close the defect entirely, especially in large leaks, because they do not often match a unique defect size or shape. To pursue total leak closure, larger or multiple devices are often used; however, it has been reported that this increases the risk of complications24. This points to the need for larger and more geometrically appropriate devices to treat this condition; in this respect, the recently introduced Occlutech PLD has increased the range of sizes and shapes available.
PERIPROCEDURAL ADVERSE EVENTS
In this series, the overall major adverse events rate (death, stroke, and emergency surgery) at 30 days was 5.6%. Similarly, Sorajja et al10 in their initial experience reported a 30-day complication rate of 5.2% (sudden and unexplained death, 1.7%; stroke, 2.6%; emergency surgery, 0.9%) in 115 patients, and Calvert et al12 described a hospital mortality of 3.9% in 259 patients. In a multivariate analysis, the only independent predictor for the combined major adverse events or mortality at 30 days was baseline NYHA functional Class IV. This result may have potential implications for the patient selection for this procedure.
Finally, prospective registries and further randomised studies are mandatory in order that percutaneous PVL closure can be offered as the first option therapy to these patients.
Limitations
This is a non-randomised, retrospective, observational study, which included centres with different volumes. There was no control group. Criteria for patient triage to percutaneous PVL closure versus re-do surgery was at the physicians’ discretion and may have varied over time, introducing bias. Long-term clinical and echo follow-up was not available for all patients. The clinical and echocardiographic results were self-reported and there was no independent adjudication. Patients treated between 2002 and 2014 were included; therefore, differences in patient selection, devices and techniques may have changed over this long time period and this may have impacted on the observed results.
Conclusion
In this multicentre, all-comers study, percutaneous PVL closure had a high procedural success and a moderate number of periprocedural complications. The type of device used and the accumulated experience of the centre were independent predictors for procedural success in mitral leaks. The leak size was the only independent predictor for procedural success in aortic leaks.
| Impact on daily practice In this multicentre experience, reflecting the largest reported “real-world” experience, percutaneous closure of paravalvular prosthetic regurgitation was performed with a reasonable rate of procedural success and a low rate of complications. These data support the fact that this procedure could be the initial therapeutic option in selected symptomatic patients at significant risk for open surgery in selected centres. The independent predictors of success and complications identified could be helpful in the selection of patients. |
Funding
I. Cruz-Gonzalez was partially supported by the “Programa de intensificacion” for researchers of Gerencia Regional de Salud, SACYL.
Conflict of interest statement
I. Cruz-Gonzalez, D. Arzamendi, X. Freixa, and A. Sanchez-Recalde are proctors for St. Jude Medical. The other authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Baseline 2D echococardiogram showing severe mitral regurgitation.
Moving image 2. Transseptal puncture.
Moving image 3. PVL crossing using a deflectable sheath (Agilis™; St. Jude Medical), a JR4 catheter and a hydrophilic straight wire.
Moving image 4. Venous-artery-wire loop by snaring the wire in the aorta and externalising it through the femoral artery.
Moving image 5. Sheath advancing over the VA loop.
Moving image 6. AVP III 10×4 mm (St. Jude Medical) deployment. An anchor wire or safety wire is left in place.
Moving image 7. Second AVP III 10×4 mm deployment.
Moving image 8. Fluoroscopic final result.
Moving image 9. Final 2D echocardiogram showing no residual mitral regurgitation.
Moving image 10. Final 3D echocardiogram showing both devices.
Supplementary data
To read the full content of this article, please download the PDF.
Second AVP III 10×4 mm deployment.
Fluoroscopic final result.
Final 2D echocardiogram showing no residual mitral regurgitation.
Baseline 2D echococardiogram showing severe mitral regurgitation.
Final 3D echocardiogram showing both devices.
Transseptal puncture.
PVL crossing using a deflectable sheath (Agilis™; St. Jude Medical), a JR4 catheter and a hydrophilic straight wire.
Venous-artery-wire loop by snaring the wire in the aorta and externalising it through the femoral artery.
Sheath advancing over the VA loop.
AVP III 10×4 mm (St. Jude Medical) deployment. An anchor wire or safety wire is left in place.

