
Abstract
Aims: Paravalvular regurgitation is an important complication of mitral valve replacement. Although surgical repair is mostly recommended, it is associated with significant morbidity. On the other hand, percutaneous closure is a less invasive alternative approach. Percutaneous approaches to treatment of paravalvular prosthetic regurgitation have emerged recently. One of them is the Occlutech Paravalvular Leak Device. The aim of this study was to evaluate early and midterm outcomes of percutaneous paravalvular leak closure utilising a novel occluder.
Methods and results: Twenty-one consecutive symptomatic patients who had moderate or severe paravalvular prosthetic regurgitation on transoesophageal echocardiography were included in the study. All the patients were clinically evaluated and found inoperable for surgery. They underwent transapical repair with the Occlutech Paravalvular Leak Device. The patients were followed for 17±5 months. Attempts were made to rectify 41 defects in 21 patients with 100% success. Mean procedure time was 76±40 min and fluoroscopy time was 44±37 min. Early post-procedural outcome was uneventful in all cases, with ≥1 grade reduction in regurgitation in all of the patients. There was no mortality during hospital stay. There was one case of haemothorax in one patient and one case of pneumothorax in another. Post-implantation 90-day follow-up data were obtained for 19 patients, and 12-month data were obtained for 12 patients. No deaths due to any cause, stroke or surgery for prosthetic impingement, worsening or relapse of paravalvular leak during follow-up were recorded. One patient underwent reintervention and was treated successfully with the same occluder 11 months after the index procedure.
Conclusions: The novel Occlutech Paravalvular Leak Device, which was designed specifically for mitral and aortic paravalvular regurgitation, is an additional, useful tool in the device armamentarium for the treatment of PVL.
Introduction
Paravalvular regurgitation is an infrequent complication of mitral valve replacement. Improper implantation of the valve, excessive annular calcification, fragile tissues at the site of ring attachment and infective endocarditis are the most important predisposing causes1. It occurs in 10% to 15% of surgically implanted heart valves2. Patients with mild or moderate paravalvular regurgitation may be asymptomatic. However, severe paravalvular regurgitation may lead to heart failure or haemolytic anaemia3. For decades, the treatment of symptomatic paravalvular prosthetic regurgitation has been open heart surgery. Percutaneous closure is a less invasive alternative, which uses a variety of complex catheter techniques to deliver occluder devices to the site of regurgitation4-8. Each treatment means carries its own risk: reoperation can entail significant morbidity and mortality, whereas percutaneous closure may be time-consuming and a second procedure may be necessary8,9. The potential for a less invasive alternative to open surgical correction has led to the development of new devices. The Occlutech® Paravalvular Leak Device (Occlutech GmbH, Istanbul, Turkey) is a novel occluder which has a unique rectangular-/square-shaped design especially designed for paravalvular leaks. We recently published two cases that were successfully treated with a novel Occlutech Paravalvular Leak Device10. Accordingly, we examined the acute and follow-up outcomes of 21 consecutive patients (including the cases of two patients previously published as case reports) treated with this novel occluder.
Materials and methods
STUDY POPULATION
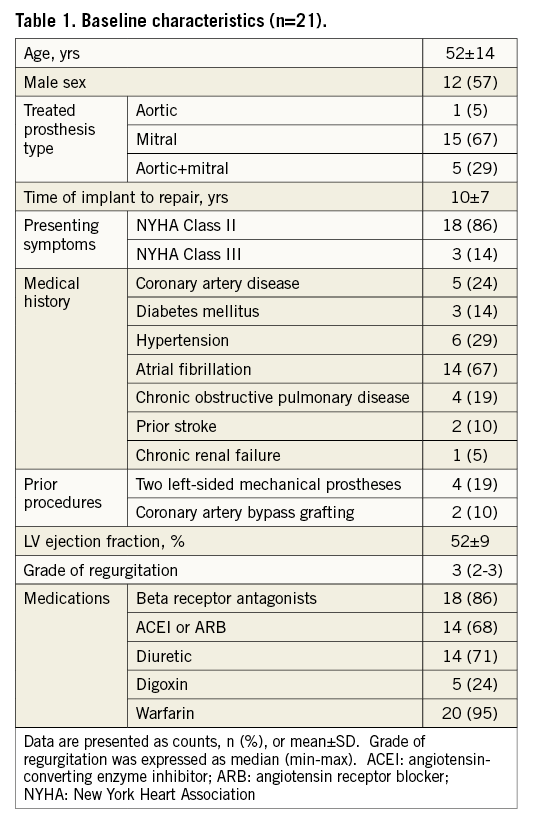
Twenty-one consecutive symptomatic patients were clinically evaluated and underwent transapical repair of paravalvular regurgitation at the Bezmialem University Department of Cardiology between June 2012 and August 2014. Inclusion criteria were the presence of severe symptoms of dyspnoea (New York Heart Association Class III or Class II with significant lifestyle or occupational impairment), and moderate or severe paravalvular prosthetic regurgitation on transoesophageal echocardiography (TEE). Patients with infective endocarditis were excluded. The procedure time was defined as the time from incision to skin closure. Every patient signed an informed consent which entailed understanding the risks associated with apical puncture, an off-label use of the device. A detailed discussion was held with every patient which included the potential therapeutic options and available data on the clinical efficacy of the transapical procedure and the device. There was routine surgical consultation. Patients could be refused due to a high morbidity and mortality risk. None of the patients declined use of their medical records for research, and the study was approved by the University Review Board.
DEVICE
The Occlutech Paravalvular Leak Device is specifically designed for paravalvular leaks. Instructions for use include compassionate use/emergency cases for non-surgical closure of paravalvular leaks (PVL). The device can be delivered via various routes (transapical and transfemoral) using small delivery catheters. The device obtained European CE mark approval on 7 October, 2014. It is made of nitinol braided mesh with a wire ranging between 67 and 107 microns according to the device size. The discs have either a rectangular frame with an ellipsoid waist or a square frame with a circular waist (Figure 1). The device is available with two types of connections between the discs, waist or twist (Figure 2). Both the rectangular and square designs have 35% lower surface areas compared to a circular design, which reduces the possibility of overlapping in case of multi-device use. There are two gold radiopaque markers at the largest part of the waists to indicate the disc frame positioning. The presence of gold markers in the device enhances visibility and allows accurate deployment and deployment across the defect. The device is available in different sizes ranging from 3 to 7 mm with a circular waist for the square device that requires 5-7 Fr sheaths and from 4×2 to 12×5 mm with an ellipsoid waist for the rectangular device that requires 5-8 Fr sheaths for delivery. Both designs are available for transapical or endovascular delivery, and both use the Flex delivery cable (Occlutech GmbH, Jena, Germany) for deployment. The Flex delivery cable does not involve a screwing mechanism; rather, the device end has a ball that fits in a socket sleeve, somewhat similar to a bioptome. Once the device end is attached to the cable end, to prevent premature release of the device, the handle of the cable is tightened. This security mechanism is activated to release the device once the device is in good position. Further information is available at the Occlutech GmbH official website (http://www.occlutech.com/images/content/products/P07F06-022-04_Occlutech%20PLD%20Br_R03b.pdf).
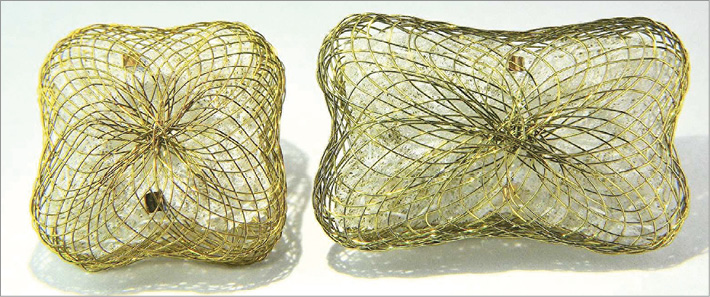
Figure 1. Square-shaped and rectangular-shaped designs of the Occlutech occluder device.
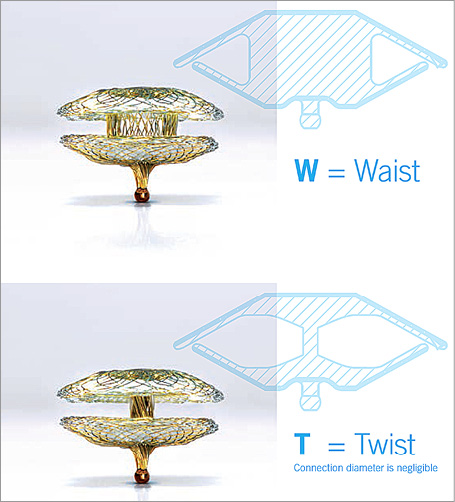
Figure 2. Side view of the Occlutech occluder device.
REPAIR TECHNIQUE
There was only a single main operator in the study (OG). A lower minithoracotomy was performed under general anaesthesia, ECG and invasive blood pressure monitoring. The apex of the heart was shown with an echocardiography machine and the area was marked before incision. The skin was incised 6 to 8 cm between the 5th or 6th intercostal space to expose the left ventricular apex. A purse-string suture was placed near the apex under direct visualisation. A heparin bolus of 5,000 U was administered intravenously. A 7 Fr short sheath (Cook Medical, Roskilde, Denmark) was inserted in the purse-string suture. A 5 Fr Judkins right catheter loaded on a superstiff 0.035” Terumo guidewire (Radifocus® Guide wire M Stiff type; Terumo Corp., Tokyo, Japan) was then introduced into the LV cavity. Under fluoroscopic and 3D TEE guidance, the guidewire was manipulated across the leak to the left atrium (paramitral leak) or aorta (para-aortic leak) according to the leak region. Then the catheter and short sheath were replaced with a 5-8 Fr Ansel delivery sheath (Cook Medical, Roskilde, Denmark). An Occlutech PVL device with a waist of the same size as the defect size was delivered perpendicular to the leak area. The decision to use single or multiple devices is determined according to the defect anatomy. A rectangular-shaped device has the advantage of being able to cover a crescent-shaped defect while avoiding interference with the valve. The design of the device is unique. It has a special braiding avoiding a distal hub, giving flexibility and adaptability with a high success rate in achieving complete closure. Both discs were initially passed through the leak for free rotation of the device. After proper alignment, the proximal disc was recaptured and then deployed in the proximal side of the leak. The position of the device and grade of leak were assessed under TEE before release. Baseline and residual regurgitation were graded semiquantitatively using Doppler echocardiography and colour-flow imaging (Figure 3, Figure 4, Moving image 1-Moving image 8). After final echocardiographic assessment, the sheath was removed, the purse-string suture was tightened and the chest was closed in the usual fashion. The patients were discharged home two to three days later on aspirin and coumadin. The indication for warfarin use was the previous surgical implantation of a prosthetic valve. Patients were invited to clinical visits one, three and 12 months after the procedure.
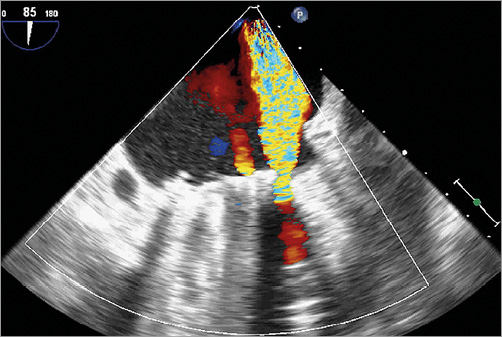
Figure 3. TEE showing severe paravalvular leak of mitral valve prosthesis.
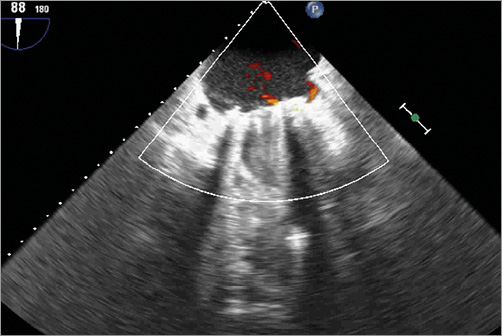
Figure 4. TEE showing no residual shunt immediately after the device has been released.
DATA ANALYSIS
Procedural success was defined as successful deployment of an occluder device that resulted in significant reduction in paravalvular regurgitation to mild or less residual regurgitation (equal to ≥1 degree in regurgitation reduction) in the absence of procedural death. Continuous variables were reported as mean±SD. Procedural and fluoroscopy times were expressed as median (min-max).
Results
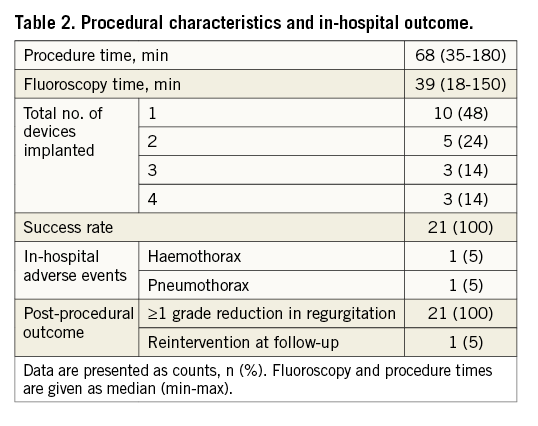
Baseline characteristics of the patients are given in Table 1: mean age was 52, and 57% of them were men. All the patients had mechanical prosthetic valves. The clinical indication for the procedure was worsening heart failure in the majority of the patients. In the study population, significant morbidity was common, with atrial fibrillation being the most frequent. Forty-one paravalvular defects were attempted in 21 patients with 100% success (Table 2). Five patients had had both aortic and mitral valve implantations, one patient had an aortic and the rest had only a mitral valve prosthesis. In eleven patients, multiple devices were placed. Five patients had two devices, three patients had three devices and three patients had four devices implanted. Two devices were implanted for para-aortic leak and the rest were implanted for paramitral leak. Mean procedural time was under 90 min and fluoroscopy time was under 60 min. There was no device embolisation, interference with the valve, pericardial effusion/tamponade, prosthetic obstruction from device deployment or need for blood transfusion. There was no mortality; however, in-hospital complications occurred in two patients. Post-implantation 90-day follow-up data were obtained for 19 patients, and 12-month data were obtained for 12 patients. No deaths due to any cause, stroke or surgery for prosthetic impingement, haemolysis, worsening or relapse of paravalvular leak during follow-up were recorded. One patient had undergone reintervention due to the appearance of a new paravalvular leak and was treated successfully with the same occluder 11 months after the index procedure.
Discussion
The current study showed that the novel Occlutech Paravalvular Leak Device, which was designed specifically for mitral and aortic paravalvular regurgitation, is a successful tool in the treatment of these patients. Transapical leak closure is a semi-invasive technique allowing direct reach to aortic and mitral paravalvular leaks. The technique is simple, easy and reliable. However, it requires a minithoracotomy, which may be a slight disadvantage in patients with severe chronic obstructive pulmonary disease and supplemental oxygen dependency. In the present study, the transapical route was used as the preference of the primary operator. The device can also be used via the retrograde approach.
Paravalvular leak has a reported incidence of 3% to 15% in different series2,11. It is usually detected in the first six months after the original procedure, but many patients remain asymptomatic and do not require further surgical intervention. The patients with symptoms often have associated heart failure and need repair. Treatment options for symptomatic paravalvular regurgitation include surgery and transcatheter repair. Although repeat operations have high mortality rates, these data were published 23 years ago and reflect the surgical outcome of cases dating back more than 30 years. There is a lack of more recently published data. It is hard to imagine that surgical reoperation results have not improved in the interim. Therefore, patient, physician and surgical preference can often influence the choice of therapy. In stable patients, repeated traditional valve replacement is the recommended treatment for paravalvular leak, which involves higher mortality rates than the original procedure, with in-hospital mortality rates of 13%, 15%, and 37% for the first, second, and third reoperations, respectively12.
The most common alternative approach remains transcatheter repair13,14. The goal of the transapical approach is to reduce the morbidity and mortality of a surgical approach while achieving good patient outcomes. Percutaneous repair of paravalvular prosthetic regurgitation can be performed with a relatively high rate of procedural success8. The largest series in the literature, performed by Sorajja et al, reached 77% acute procedural success, with an acceptable incidence of major adverse clinical events (8.7%)8. Although the number of patients in our cohort was limited, it should be noted that the acute procedural success rate in the current study was 100%. The most common limitation of percutaneous repair is the difficulty in placement of delivery sheaths and catheters across the lesions. Bleeding complications are also relatively high. Prosthetic impingement is another major potential complication of percutaneous repair. Therefore, the feasibility for percutaneous closure has to be assessed by defining the shape, size and location of the defect. Other possible adverse outcomes after catheter repair of paravalvular leaks include stroke, arrhythmia, cardiac perforation, late device dislodgement and renal failure8,15. The Paravalvular Leak Device shows its efficacy in these types of defect, completely recovering the regurgitant orifice. Rectangular-shaped devices are especially useful for edge repair in crescentic leaks, whereas square-shaped devices seal the centre of the regurgitant orifice. In cases with large crescent-shaped defects requiring multiple devices, implantation of rectangular devices to the edges increases the success rate and lowers residual regurgitation without interfering with the valve. However, in our series there was still a need for multiple devices in order to cover the edges of several crescentic defects completely.
Randomised controlled studies comparing transfemoral and transapical paravalvular leak repair have not been constructed yet. Only five cases have been reported in the literature describing the transapical approach with the AMPLATZER™ Septal Occluder device (St. Jude Medical, St. Paul, MN, USA)16-18. No mortality or intraoperative complications were recorded. However, device instability and severe mitral regurgitation were detected in the latter case. It should be noted that AMPLATZER occluders are not specifically designed for paravalvular leak repair and there is always a risk that the waist of the device may exacerbate the regurgitation due to the thicker waist and circular design. The Occlutech Paravalvular Leak Device has a specifically designed rectangular or square shape to overcome this important problem. Another advantage of this novel device is that it allows total repositioning/realignment which helps the operator to close the leak successfully. The transapical technique also has several advantages. Access and positioning of the devices can be performed more precisely and easily than in a transfemoral approach. The transapical approach is the preferred approach of the operator in the current study. It allows straight access especially for paravalvular mitral defects, which was the situation of all of the patients in this series. However, patients should be closely screened for potential complications related to the procedure (e.g., haemothorax and pneumothorax, which occurred in one patient each in our series). In a recently published study, transapical access was shown to be a safe way of access with a complication rate of 7.1%19.
A team approach employing anaesthesiology, cardiovascular surgery, 3D echocardiography and invasive cardiology experts is extremely important in paravalvular leak repair. Echocardiography with 3D defect reconstruction is the gold standard for guiding leak closure. TEE enables the detection of complications and confirms correct canalisation of the leak with the catheter and the position of the device20. Therefore, a 3D echocardiographer is a prerequisite in paravalvular leak repair. Furthermore, team training, a visit to an experienced centre to view an actual implantation and performing the first procedures with an experienced proctor are mandatory.
Finally, it should be noted that our cohort was fairly young and healthy, at a mean age of 52 with only one prior thoracotomy and little comorbidity. It is well known that clinical and demographic characteristics of patients are related to the success and complication rates. Although the interventional decisions can only be made on a case-by-case basis, in other surgical centres some of these patients might have been deemed operable.
Conclusion
The novel Occlutech Paravalvular Leak Device, which was designed specifically for mitral and aortic paravalvular regurgitation, is an additional, useful tool in the device armamentarium for the treatment of PVL.
| Impact on daily practice The novel Occlutech Paravalvular Leak Device, which was designed specifically for mitral and aortic paravalvular regurgitation, can be used with confidence in the treatment of these patients. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. TEE showing severe paravalvular leak of mitral valve prosthesis.
Moving image 2. 3D echocardiography showing severe paravalvular leak at 5 to 9 o’clock position.
Moving image 3. 3D echocardiography showing placement of an occluder at 6 o’clock position.
Moving image 4. 3D echocardiography showing successful deployment of a second occluder at 7 o’clock position.
Moving image 5. 3D echocardiography showing placement of an occluder at 9 o’clock position.
Moving image 6. 3D echocardiography showing successful deployment of three occluders side by side.
Moving image 7. Fluoroscopic image after successful deployment of the occluders.
Moving image 8. TEE showing no residual shunt immediately after successful deployment of the occluders.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. TEE showing severe paravalvular leak of mitral valve prosthesis.
Moving image 2. 3D echocardiography showing severe paravalvular leak at 5 to 9 o’clock position.
Moving image 3. 3D echocardiography showing placement of an occluder at 6 o’clock position.
Moving image 4. 3D echocardiography showing successful deployment of a second occluder at 7 o’clock position.
Moving image 5. 3D echocardiography showing placement of an occluder at 9 o’clock position.
Moving image 6. 3D echocardiography showing successful deployment of three occluders side by side.
Moving image 7. Fluoroscopic image after successful deployment of the occluders.
Moving image 8. TEE showing no residual shunt immediately after successful deployment of the occluders.

