
Abstract
Aims: We sought to evaluate the feasibility and efficacy of hybrid transapical closure of paravalvar mitral leaks using a new Occlutech PLD occluder in patients with heart failure and/or haemolytic anaemia.
Methods and results: Retrospective analysis of clinical and procedural data was undertaken for patients who had attempted closure of paravalvar mitral leaks via a hybrid transapical approach with the Occlutech PLD occluder. Eight patients (four males, median age 69 years) underwent closure of 10 mitral paravalvar leaks using eight Occlutech PLD occluders and two AMPLATZER Vascular Plugs (AVP II). Successful deployment, with significant reduction of the paravalvar leak was achieved in seven patients with short procedure (median 131 min) and fluoroscopy times (median 22 min). One patient had mechanical interference with prosthetic valve function, requiring surgery. Another patient with a high EuroSCORE (48.8%) died of multi-organ failure two days after the procedure. Clinical improvement in either heart failure or haemolysis was seen in all discharged patients.
Conclusions: In our series of patients with challenging anatomy, the Occlutech PLD occluders performed well when implanted via a hybrid transapical approach. Further work is needed to assess this methodology fully for a wider population and to assess other deployment approaches for this promising new occluder.
Introduction
Periprosthetic paravalvar leaks (PVL) occur as a result of limited dehiscence between the native valve annulus and the sewing ring of a prosthetic valve and may occur in seven to 17% of patients following valve replacement1,2. A minority of patients with paravalvar leaks may become symptomatic with congestive heart failure and/or haemolysis.
Medical management of paravalvar leaks offers some symptomatic benefit, whilst conventional surgical repair may be associated with a high surgical morbidity and a high risk of recurrence3. Since 19924, and particularly over the last decade, vascular plugs, duct occluders, and atrial and ventricular septal defect closure devices have been increasingly used to occlude these leaks with variable immediate and short-term clinical outcomes5-10. These devices are not designed specifically for paravalvar leak occlusion, and procedural outcomes of residual leaks and impingement on valve function reflect this11.
The approach for device closure of mitral paravalvar leaks can be percutaneous through a transseptal12 or direct apical puncture13,14, or a hybrid surgical/interventional approach can be adopted15-18. The advantages of the transapical approach include the ability to access directly the entire mitral annulus, making medial PVLs easier to deal with.
Given the anatomy of the mitral annulus, the majority of paravalvar leaks are crescent-shaped and the new Occlutech® PLD occluder (Occlutech GmbH, Jena, Germany)16, available in rectangular shapes, appears to be well suited for effective closure of these leaks. We report on the feasibility and efficacy of this new device through a hybrid transapical approach.
Methods
PATIENTS
Patients with mitral paravalvar regurgitation, associated with congestive heart failure and/or haemolysis were discussed in a multidisciplinary team meeting and considered for device closure of PVL. All eight patients included in this retrospective analysis had mechanical mitral prostheses: seven had bileaflet tilting disc prostheses and one had a single leaflet tilting disc prosthesis. Written approval was obtained from the Medicines and Healthcare Products Regulatory Agency (MHRA) for the compassionate use of the (then) non-CE-marked Occlutech PLD device.
DEFINITIONS
Paravalvar regurgitation was defined as regurgitation arising between the native annulus and the sewing ring of the prosthetic valve, identified on transthoracic echocardiography and confirmed on transoesophageal echocardiography (TOE). Baseline risk was calculated using the logistic EuroSCORE19 (http://www.EuroSCORE.org/calc.html) and the Society of Thoracic Surgeons (STS)20 (http://209.220.160.181/STSWebRiskCalc261) scores using online calculators. Prolonged ventilation was defined as ventilation for more than 24 hours. A long hospital stay was defined as a stay longer than 14 days.
IMAGING
All patients had pre-procedural transthoracic (TTE) and 3D transoesophageal (TOE) echocardiography to assess the number, size, position and severity of the paravalvar regurgitation. The location of the leak was described using a clock face from a surgical view, viewing the mitral valve from the left atrial aspect, with 12 o’clock corresponding to the area of aortomitral continuity, three o’clock close to the atrial septum, six o’clock at the left atrial posterolateral free wall and nine o’clock near the left atrial appendage (Figure 1). The size of the leak was assessed at the vena contracta on 3D colour and multiplanar reconstruction (MPR) imaging.
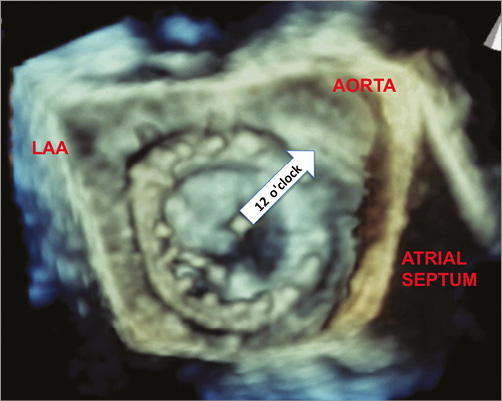
Figure 1. Left atrial (surgical) view of a mechanical mitral valve. 3D transoesophageal echocardiographic image of the left atrial view of the mechanical mitral valve showing the anatomical landmarks and reference points for paravalvar leaks. The area of aortomitral continuity is 12 o’clock on the clock face.
THE OCCLUTECH PLD PARAVALVAR LEAK OCCLUDER
The Occlutech PLD occluder consists of a nitinol wire mesh and has two main configurations, square and rectangular with varying disc and waist sizes (Figure 2). A flexible circular (square device) or oval (rectangular device) waist connects two adjacent retention discs which contain two thin polyethylene patches. Gold markers on the device are oriented along the short axis of the rectangular device. The waist sizes range between 4 mm and 7 mm for square devices and from 4×2 mm to 18×10 mm for the rectangular devices. Rectangular devices with waist sizes larger than 12×5 mm were developed in the latter part of the study. The left atrial disc (distal) is larger than the left ventricular disc (proximal) on the attachment side of the device by 1.5 to 2.5 mm (Table 1). These devices can be used in the mitral and aortic positions, and can be delivered via a femoral venous or arterial or transapical approach.
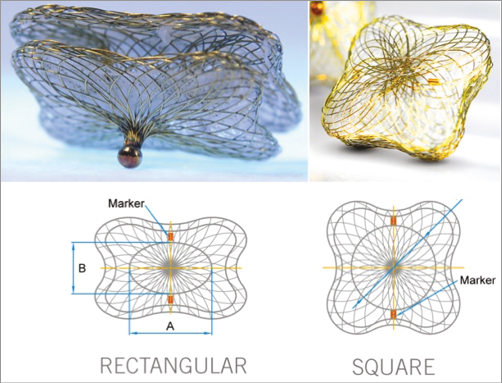
Figure 2. The Occlutech PLD device. The Occlutech PLD rectangular (left) and square (right) occluders. The waist is oval in rectangular devices and round in square devices. Gold markers are oriented along the short axis of the rectangular device (diameter B).
PROCEDURE
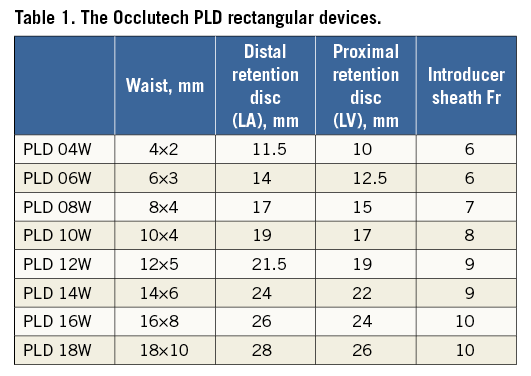
All the procedures were performed under general anaesthesia. A limited thoracotomy was performed to expose the apex and two apical purse-string sutures were placed, followed by insertion and fixation of one or two short sheaths, depending on the size and number of leaks identified on TOE (Figure 3). Under fluoroscopy and TOE guidance, the leak was crossed with a hydrophilic guidewire and a variety of end-hole catheters. Successful crossing of the paravalvar leak was confirmed on echocardiography and confirmed also on fluoroscopy. The hydrophilic guidewire was exchanged for a stiff guidewire, followed by placement of a 55 cm long Flexor® sheath (Cook Medical, Bloomington, IN, USA) through the apical short sheath. Device selection was based on the morphology and size of the defect, with rectangular devices used for the crescentic larger defects and square devices for the smaller circular defects. In the first four patients, the largest Occlutech PLD occluder available was one with a waist of 12×5 mm: this was used in defects that were slightly larger, relying on the retention discs to achieve the seal. The selected occluder was introduced through the long sheath. Positioning and deployment of the LA disc, the waist and the LV discs were performed with fluoroscopic and transoesophageal echocardiography. With the rectangular device, orienting the long axis of the device with the annulus was achieved through TOE guidance. With the device in position, valve function and residual leaks were assessed prior to and after release of the device. Upon satisfactory deployment and release of the device, the sheaths were removed and the apical purse-string sutures tied for haemostasis of the apical access site (Figure 3-Figure 5).
Figure 3. Procedural steps. Initial surgical steps including apical thoracotomy (A), apical purse-string suture (B) and sheath placement in the LV apex (C). The rectangular Occlutech PLD device with a waist between the two retention discs (D), attachment to the delivery cable (E) and preparing to withdraw the device into the sheath (F).
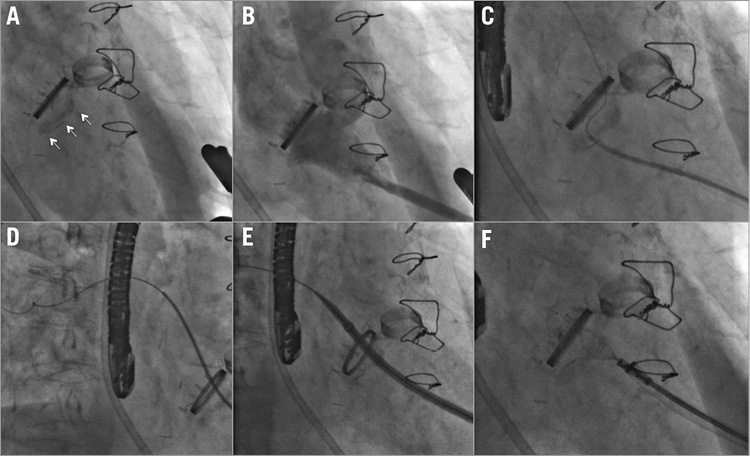
Figure 4. Procedural steps on fluoroscopy. A) The rings of the mechanical mitral and aortic valves with mitral annular calcification (arrows). B) Left ventricular angiogram showing opacification of the left atrium through the paravalvar leak. C) Hydrophilic wire crossing the paravalvar leak. D) Distal wire and catheter position in the pulmonary veins. E) Hydrophilic (Flexor) sheath advanced to the left atrium. F) Occlutech PLD device positioned across the paravalvar leak.
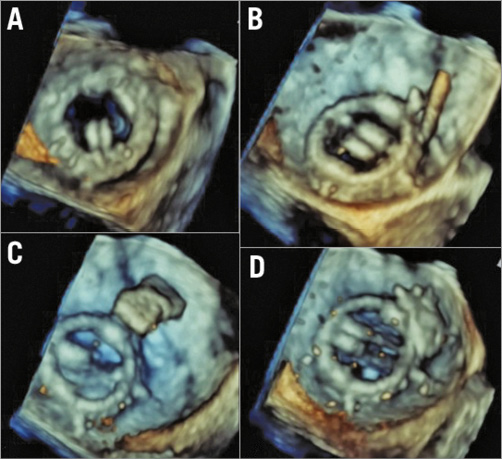
Figure 5. Procedural steps on transoesophageal echocardiography. A) Live 3D images during the paravalvar leak closure showing a medial paravalvar leak, crescentic in shape. B) Successful crossing of the leak with a hydrophilic wire.C) The rectangular Occlutech device in the mid-left atrium but perpendicular to the valve annulus. D) The device rotated into a more favourable position as it was withdrawn towards the mitral prosthesis.
Prior to discharge, all patients had the following: chest X-ray to confirm full lung expansion and device position, TTE to assess any residual leaks, device location and prosthetic valve function, and an electrocardiogram (ECG) to document rhythm and any conduction abnormalities. The patients remained in hospital until a therapeutic international normalised ratio (INR) was achieved.
At follow-up, all patients had a clinical evaluation, ECG and TTE. Further tests, including blood tests and TOE, were performed as clinically indicated.
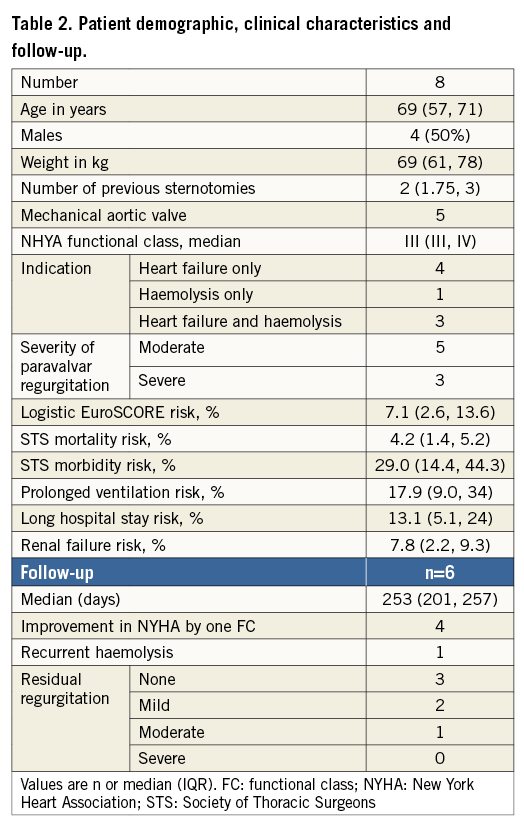
Eight patients, all with mechanical mitral valve prostheses (seven with bileaflet tilting disc prostheses) with 10 paravalvar leaks, were included in this retrospective analysis. The baseline demographic and clinical characteristics of the patients are described in Table 2. Uncomplicated transapical access was possible in all eight patients, with the insertion of a single short sheath in seven patients and two sheaths in one patient with two significant leaks. The use of two sheaths in this patient was to allow simultaneous delivery and evaluation of both devices before their release.
Results
TECHNICAL SUCCESS
Ten devices were used to close the ten paravalvar leaks in eight patients, with a median procedure time of 131 minutes (IQR 109, 139), fluoroscopy time 22 minutes (IQR 16, 30) and radiation dose of 5,388 cGycm2 (IQR 2,960, 7,133). Devices deployed and released were: four 12×5 mm, two 7×7 mm, one 16×8 mm, one 18×10 mm Occlutech PLD occluders, one 12 mm and one 14 mm AMPLATZER™ Vascular Plug (AVP II) (St. Jude Medical, St. Paul, MN, USA). Successful closure, defined as no interference with prosthetic valve function and improvement of the leak by at least one grade, was possible in seven of the eight patients (Table 3). The patient in whom successful closure was not possible had a single tilting disc mechanical prosthesis, two PVLs, a large leak (five-seven o’clock) and smaller anterior leak (11 o’clock) causing slight rocking of the valve prosthesis. A rectangular 12×5 mm Occlutech PLD device was placed in the large leak and a square 7×7 mm Occlutech PLD device was placed in the anterior leak. When positioned, the square device impinged on the valve leaflets, but leaflet motion normalised on repositioning and the devices were released. TTE and TOE the following day showed re-impingement on the valve leaflet by the square Occlutech PLD device and significant valvar regurgitation. We postulate that, due to the large circumference of the mitral annulus occupied by the PVL and the rocking movement, the square device moved overnight. The patient underwent surgery three days later to remove the two devices and repair the paravalvar leaks. Successful closure was possible in the remaining patients. However, in one patient with significant mitral annular calcification, attempted closure with two Occlutech PLD devices (14×6 mm and 12×5 mm) repeatedly interfered with the tilting discs. A 14 mm AVP II was successfully used instead, as the waist between the two retention discs was longer, allowing the annular calcification to be cleared.
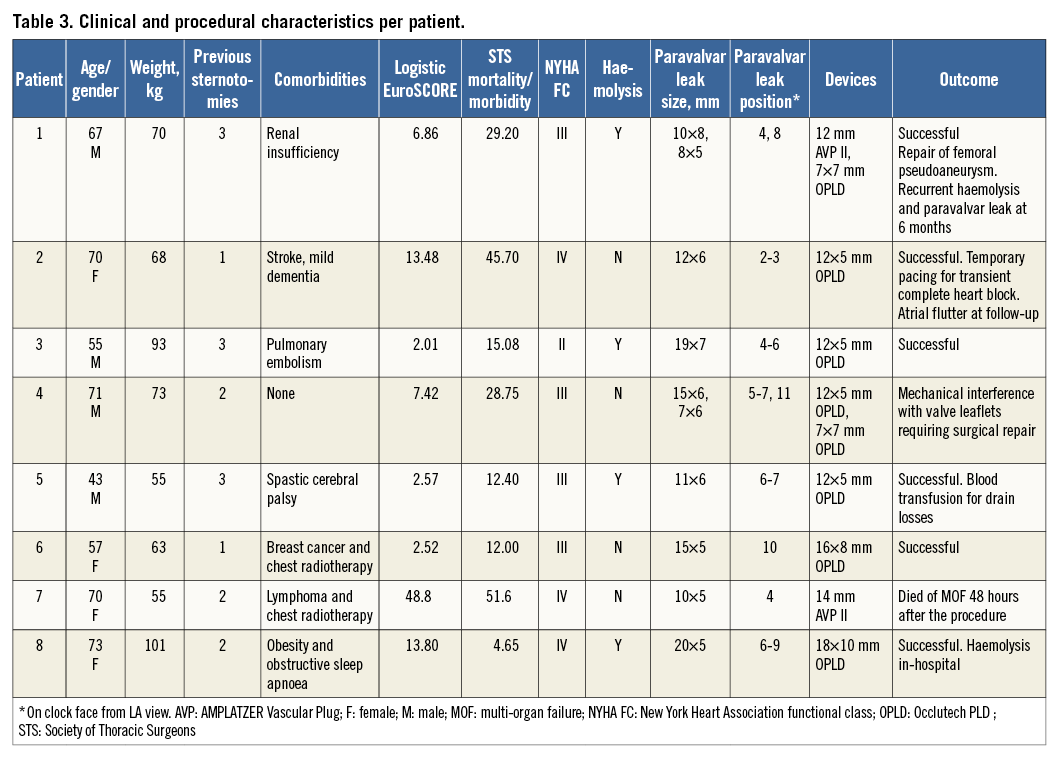
MORTALITY AND COMPLICATIONS
One patient, with a logistic EuroSCORE of 48.8%, died 48 hours post procedure from multi-organ failure. This patient was in the intensive care unit and the procedure was performed as a last resort following clinical deterioration and a protracted intensive care stay after previous mechanical mitral valve replacement. The transapical PVL closure in this patient was technically successful (using a 14 mm AVP II device), reducing the degree of regurgitation and not interfering with leaflet motion, but the multi-organ failure was progressive.
The patient in whom the device interfered with a single leaflet tilting disc underwent surgery to remove the Occlutech PLD occluders and surgically repair the paravalvar leaks with a good outcome. One patient, who had a 5 Fr sheath inserted in the right femoral vein for potential transseptal puncture, developed an arteriovenous fistula requiring surgical repair. Two patients developed reversible acute kidney injury, not requiring dialysis. One patient, with pre-existing trifascicular block developed transient complete heart block on induction of a general anaesthetic, requiring temporary transvenous pacing during the procedure, followed by 24 hours of epicardial pacing after the procedure. Atrioventricular conduction recovered and the patient was discharged without a permanent pacemaker.
Excluding the patient who was ventilated prior to the procedure, none of the remaining patients required prolonged mechanical ventilation (more than 24 hours). Two patients required a blood transfusion after the procedure, one for significant drain losses following the initiation of heparin and another for ongoing haemolysis.
Haemolysis improved in all but one patient immediately after the procedure. This patient had a moderate PVL around a bioprosthetic tricuspid valve in addition to the mitral leak, and the latter was successfully occluded. Haemolysis was not seen as a new event in any of the patients.
The median hospital stay after the procedure in the surviving patients was 11 days (range four to 26 days), with only two patients having a hospital stay exceeding 14 days. This included the time needed to achieve therapeutic anticoagulation and safe discharge in some of the frail patients. Echocardiography prior to discharge showed resolution of the PVL in three out of six patients and improvement in the degree of PVL to mild in the remaining three. There were no device embolisations or periprocedural strokes.
FOLLOW-UP
During a median follow-up of eight months, all six patients who were discharged from the hospital after a successful hybrid PVL closure were alive. Four patients had an improvement of their NYHA functional status by at least one class. Of the two patients who did not show an improvement in their functional class, one had the procedure for haemolysis and, although the haemolysis resolved, the patient remained in NYHA Class II. The second patient had cerebral palsy and at initial presentation had both haemolysis and heart failure (NYHA Class III). His mobility was additionally limited by the cerebral palsy and he remained in NYHA Class III, even though the haemolysis resolved. Follow-up echocardiography showed the devices in situ in all patients, with a sustained significant improvement in the degree of mitral regurgitation in five patients. One patient with two PVLs, who underwent the procedure for heart failure and haemolysis, had recurrence of haemolysis at six months, with TOE confirming a residual moderate PVL around both devices (12 mm AVP and 7×7 mm Occlutech PLD). One patient developed an atrial flutter requiring cardioversion at follow-up (Table 2).
Discussion
Our study is the largest reported series of patients in whom the new Occlutech PLD occluder was used to close mitral paravalvar leaks using a hybrid transapical approach. In our patients, this approach to closing the leaks using this device was an efficacious and feasible alternative to surgical or percutaneous transseptal closure. The larger rectangular Occlutech PLD devices allow attempts at closure of the larger, crescentic paravalvar leaks, for which other occluders may not be particularly suited because of their size and shape.
The Occlutech PLD device is specifically designed for closure of large paravalvar leaks. Goktekin et al reported the initial results on “first-in-man” use in 201416. Paravalvar leak closure with other nitinol mesh devices, designed and approved for closure of other communications and not PVLs, is associated with modest procedural success rates of 30-80%21,22, as well as longer-term risks of progressive residual leaks, fractures and erosions23.
In recent years, the AMPLATZER Vascular Plug III (AVP III) device (St. Jude Medical) has been increasingly used for PVL closure given its oblong shape24. Compared with the Occlutech PLD device, the AVP III device is available in smaller sizes (largest long-axis diameter of 14 mm compared with the Occlutech range of 11.5 to 28 mm), and has narrower overhanging rims. Unless multiple AVP III devices are used, it is only suitable for small PVLs. The design and particularly the larger size ranges of the rectangular Occlutech PLD device are suited to the most frequently encountered large crescentic leaks in symptomatic patients (six out of ten devices used in our patients were rectangular with a minimum disc size of 21.5/19 mm and a waist of 12×5 mm). Although large leaks may be closed with multiple currently available devices, one of the factors that may contribute to embolisation of devices is closure of a large leak with multiple devices. In our cases, given the available large sizes of rectangular devices, none of the individual leaks needed more than one device for occlusion, minimising the risk of device embolisation. Despite a self-selected group of patients with challenging anatomical features, we have demonstrated the efficacy of this device and the value and role of the hybrid approach.
TRANSAPICAL APPROACH
The transapical approach is particularly suitable for PVLs close to the atrial septum. However, it is also useful in lateral leaks in the presence of a mechanical prosthetic aortic valve, making the establishment of a guidewire circuit through the aortic valve impossible. Positioning the guidewire in a pulmonary vein through the apical approach lends itself to good stability of the sheaths. Given the positions of the leaks and the high prevalence of mechanical aortic valves, all our cases were planned for transapical surgical access. In our experience, fluoroscopy times (22 minutes) were shorter than those reported by Ruiz et al9 (42.6 minutes) for mitral PVL closure. Our total procedure time of 111 minutes was also shorter than that reported in the large series by Rihal et al8,12 of 147 minutes in 115 patients (78% paramitral leaks), mostly using a transseptal approach. The advantage of the transseptal approach is the avoidance of a thoracotomy; however, in challenging locations of PVLs, a hybrid approach has advantages. Therefore, both approaches should be seen as being complementary, and the access planned on a case-by-case basis.
OPERATIVE RISK
Our cohort of patients represents a high-risk group for perioperative complications and morbidity, 75% having had at least two previous sternotomies. Despite a 14% STS risk for prolonged ventilation, all six patients attending electively for the procedure and who had successful closure were extubated and stepped down from intensive care within 24 hours. Swaans et al reported an average hospital stay of five days in patients undergoing PVL closure through a similar approach, which is significantly shorter than our post-procedural average stay of 11 days15. All our patients had mechanical valves, compared with only half the patients in the group of patients from Swaans, requiring a longer stay to achieve therapeutic anticoagulation. Different complications and social factors are also contributory to this length of stay.
UNSUCCESSFUL CLOSURE - FACTORS
In our cohort, all paravalvar leaks could be accessed through the transapical approach, and failure to close the leak was related to anatomical factors. Thus, successful closure of the mitral paravalvar leak using the Occlutech PLD device was possible in 75% of patients. The factors that may contribute to difficult and unsuccessful closure include the following. 1) Mitral annular calcification (MAC): a degree of mitral annular calcification is common in elderly patients and is a predisposing factor for PVL. If severe, as was the case in one of our patients, this may interfere with the deployment of the left ventricular disc, which, in turn, may interfere with leaflet motion of the prosthetic valve. The severity of the MAC, as well as its relationship to the PVL, should be considered when planning these procedures. In this situation, the use of alternative devices (e.g., vascular plugs) with a greater waist depth and distance between the left atrial and left ventricular discs, may allow the discs to “clear” the MAC and therefore interfere less with the leaflet motion. 2) Single versus bileaflet tilting discs: the one patient in our cohort who had a single leaflet tilting disc prosthesis had impingement on the valve leaflet by the PLD device. This patient had a large paravalvar leak and a rocking valve, which may have been just as important a contributory factor. 3) Size of the leak and stability of the valve: with very large paravalvar leaks and dehiscence of a large circumference of the annulus, manoeuvring sheaths and devices around the valve can lead to further instability.
DEVICE CHOICE
The Occlutech PLD devices, both in square and rectangular shapes, add to the already existing range of devices used for transcatheter PVL closure. Compared with other rectangular/oblong devices such as the AVP III, the Occlutech PLD device is available in larger sizes, of both waist and overhanging rims. This allows large defects to be sealed with a single device. These devices may not be suitable for all ranges of leaks, and so a range of other occluders needs to be available, as in our case with severe mitral annular calcification needing a device with a longer waist.
Conclusion
The Occlutech range of PLD occluders is designed to tackle a large range of paravalvar leak sizes and morphologies. In our series of cases with particularly challenging anatomy, the devices performed well when implanted via a hybrid transapical approach. Further work is needed to assess this technique fully for a wider population of paravalvar leak cases and also to assess other deployment approaches for this promising new occluder.
| Impact on daily practice The Occlutech range of PLD occluders is designed to tackle a large range of paravalvar leak sizes and morphologies, and adds to the range of devices used for transcatheter PVL closure. The possibility of impingement on the prosthetic valve discs should be considered in the presence of single leaflet prostheses and significant mitral annular calcification. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

