Abstract
Aims: To understand the incidence, aetiology and mechanisms of paravalvular aortic and mitral leaks after valvular surgery; reviewing the best methods for diagnosis, procedural guidance and result assessment of these leaks, as well as describing the different approaches to their treatment.
Methods and results: A literature search was undertaken as well as an in-depth analysis of our own experience concerning different imaging modalities and various therapeutic strategies for aortic and mitral paravalvular leaks. The majority of patients were diagnosed using two- or three-dimensional transoesophageal echocardiography, useful in both guiding the procedure as well as assessing the procedural results. Haemoglobin, haematocrit, LDH and haptoglobin values were analysed to assess haemolysis. Procedural success for percutaneous closure of paravalvular aortic leaks are around 90% in the different series, with low complication rates. Mitral leaks have been approached by transfemoral and transapical access; the reported success of this procedure ranges from 75% to more than 90% in different reports. Complication rates at 30 days average 10% and mortality related to the procedure is around 1%. Late follow-up results depend on the initial anatomy, baseline clinical class and procedure results.
Conclusions: Paravalvular leaks after surgical valve implantation have a multifactorial aetiology, but are mainly related to specific anatomic characteristics of the valvular ring. Mitral leaks are three times more common than aortic leaks and the incidence increases after reoperation. Different percutaneous techniques with several devices have been explored for leak closure, but we are still lacking devices specifically designed to treat this pathology more effectively.
Abbreviations
Ao: aorta
AV: arteriovenous
AVP III: Amplatzer Vascular Plug III
FC: functional class
LA: left atrium
LDH: lactate dehydrogenase
LV: left ventricle
NYHA: New York Heart Association
PVL: paravalvular leak
RA: right atrium
TEE: transoesophageal echocardiography
Introduction
Paravalvular leak (PVL) is a complication of valve replacement surgery due to incomplete apposition of the sewing ring to the native tissue1. Small PVLs may be detected in almost half of the patients who have undergone surgical valve replacement2,3. Most PVLs remain asymptomatic; however, 1-5% of the patients with prosthetic valves present clinically significant PVLs3,4.
Surgical reoperation is the gold standard therapy for symptomatic PVLs, but it is associated with significant mortality and morbidity5. Transcatheter closure of PVLs has been performed in selected high-risk patients in a relatively small number of centres for more than 20 years. However, only a limited number of case reports and relatively small series of patients have been reported, describing results from different centres using various types of device6-16.
Presently, there are no devices available that are specifically designed to meet the anatomic and physiologic properties of PVLs. Rather, devices that have been approved for the closure of other cardiovascular defects are used in an off-label fashion.
PATHOGENESIS
PVLs are due to incomplete apposition of the sewing ring to the native tissue1: this problem is generally the consequence of suture dehiscence and is related to annular calcium, friability of the annulus tissue, lack of space to locate the valve sutures, localised infection, the ring not being circular (circular prosthetics) and technical difficulties in accessing the ring valve tissue2,14-19. Furthermore, in patients with PVLs, new PVLs are observed at follow-up in other places in the same valve ring20.
The paravalvular defect produces regurgitation, generating volume overload and damage to red cells while crossing the PVLs, secondary to the shear stress resulting in heart failure and haemolytic anaemia21.
INCIDENCE
The real incidence is unknown and the reported incidence in different registries varies widely, ranging from 1.5% to 47%: Ionescu reported 6% incidence in the aortic valve and 32% in mitral valves, and seen as being more common in mechanical valves20. Rallidi observed PVLs in 47%; the majority (90%) were small, with a benign clinical course2. In Jindani’s report, the incidence was 7%15. Wasowicz found 15% in 442 patients22. Genoni studied 76 patients with mitral PVLs and found that the size was small (1-2 mm) in 43%, intermediate (3-5 mm) in 27%, and large (6-15 mm) in 30% of patients, more frequent in patients with renal disease23. In 3,201 consecutive patients who underwent isolated standard aortic valve replacement Sponga observed an incidence of 4.2%24. In 256 patients with aortic valve replacement with stentless bioprostheses, Beholz reported an incidence of 1.37%/patient/year25.
Diagnosis
CLINICAL PRESENTATION
In most cases patients with PVLs are asymptomatic. In 1-5% of patients with prosthetic valves, a larger leak of clinical significance may be apparent2,14,15. Clinically significant PVLs most often occur in association with mitral prostheses, less often with aortic, and only rarely with pulmonary or tricuspid prostheses26.
The symptoms are dyspnoea, and fatigue secondary to congestive heart failure and haemolytic anaemia. The New York Heart Association (NYHA) functional class is related to the degree of valve regurgitation and the size of the defect, and the numbers of leaks are related to haemolytic anaemia23. The destruction of red cells increases LDH and decrease haptoglobin15,27,28.
On the other hand the symptoms of congestive heart failure may be related to a different valvular problem such as tricuspid regurgitation, to pulmonary hypertension or to left and/or right ventricular dysfunction.
ECHOCARDIOGRAPHY
Transoesophageal echocardiography (TEE), especially 3-D, is the gold standard diagnostic method. It is very useful to define the location, shape and severity of periprosthetic regurgitation, facilitating the design and guiding of the procedure29.
With respect to localisation, aortic PVLs are classified in relation to the sinuses of Valsalva (right, left and non-coronary)30. Mitral PVLs are classified with respect to the aortic valve (Figure 1)31.
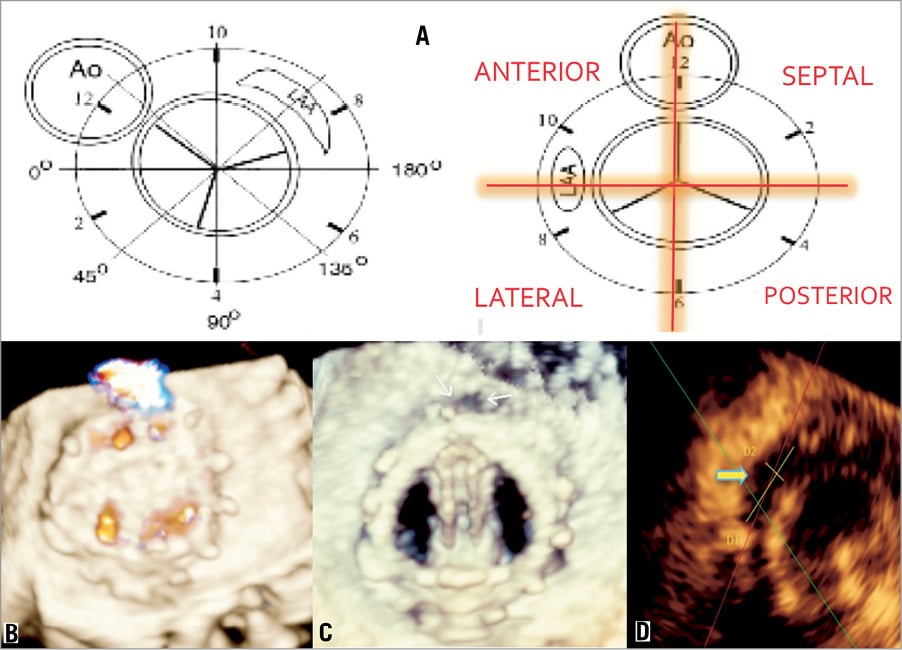
Figure 1. Mitral valve prosthetics, echocardiographic description of locations. A) Localisation of leaks, defects related to the aorta and left atrial appendage31. B) and C) show 3-D TEE of a mitral prosthetic valve as well as leaks in the anterior-septal position 0º. D) Estimating the leak size.
TEE 3-D perfectly defines the size and shape of the PVLs, especially mitral PVLs32. The course of a PVL is often difficult to define, especially in very calcified leaks33.
It is often difficult to assess the grade of regurgitation, several haemodynamic parameters often being required34.
In some reports, intracardiac echocardiography has been used to guide the procedure35.
During the procedure, 3-D TEE helps to cross the wire through the leak, to assess the degree of regurgitation after the wire and sheath have crossed the defect, and to choose the adequate device36-38. After implanting the device, TEE confirms the correct functioning of the prosthetic valve, the correct positioning of the device, and the degree of residual regurgitation if any36,39,40.
At follow-up, 3-D TEE is very useful to evaluate device status, thrombus, residual regurgitation and the appearance of new PVLs12,34,41,42.
ANGIOGRAPHY
Contrast angiography is a useful method to make the diagnosis, to define the location and to estimate the size in cases of aortic PVLs: a PVL located in the right sinus of Valsalva is best visualised in the left anterior oblique view, those located in the left coronary sinus in the right anterior oblique view, and non-coronary PVLs in the lateral view. In the case of mitral PVLs, the utility of angiography is less well established; however, anterolateral PVLs are best approached with fluoroscopy in the posteroanterior view with cranial angulation, posteroseptal PVLs in the right anterior view, and lateral PVLs in the lateral view. It is important to use the least possible amount of contrast in these patients with congestive heart failure.
MRI
This technique estimates the flow-imaging and volume-based measurements showing high-grade paravalvular leakage. It can accurately assess periprosthetic valve leakage with multiple regurgitation jets43.
CT WITH 3-D/4-D
Reconstruction with volume rendering of pre-acquired CTA images allows identification of PVLs on the reconstructed image, and helps to identify the best projection to cross the wire. It is also very useful to identify the apex and to define the course of the left anterior descending artery in cases of transapical approach12.
Treatment and prognosis
The majority of patients are asymptomatic; only 1-5% present with heart failure and/or haemolytic anaemia. The indications for re-intervention are not yet universally accepted44,45. The severity of symptoms and degree of paravalvular regurgitation (moderate to severe) are accepted as indications for reintervention44.
Medical management of heart failure and haemolytic anaemia with red blood cell transfusion, eritropoyetin, intravenous iron, or folic acid is of limited value.
Traditionally, a surgical approach with new reintervention valve replacement has been the accepted treatment. However, reoperation is associated with higher mortality and morbidity than the initial operation, with reported mortality ranging from 6% to 42%, and important morbidity which includes, among others, perioperative stroke 5.1%, sternotomy wound infections and reconstruction 25%, long hospital stay and recurrence of the leak in the same place in the range of 20-37%44-50. For these reasons, patients with paravalvular leaks are considered high-surgical-risk or inoperable, and percutaneous techniques have been developed in an attempt to repair them.
PERCUTANEOUS APPROACH
PRE-PROCEDURE
Before the procedure, a lab evaluation of haemolysis including blood cell count, indirect bilirubin, LDH and presence of schistocytes and reticulocytes in blood smears is recommended. A complete evaluation of the heart by TEE, focusing especially on the PVL and mechanical valve(s), should be done immediately before the procedure. Formal contraindications to percutaneous repair should be analysed, including instability of the prosthesis, evaluating the risk of movement of the prosthesis, active endocarditis and/or presence of thrombi. Coumadin should be replaced by subcutaneous heparin two to three days before the procedure and antibiotic prophylaxis should be administered before the procedure. The preferred access to perform the procedure (transfemoral or transapical) should be evaluated.
TRANSAPICAL ACCESS
Transfemoral access is the simplest and most widely used approach for the closure of paravalvular leaks. Nevertheless, the transapical approach could be a good alternative for patients with severe transfemoral access difficulties, in those where closure is not possible via transfemoral access, or for mitral leaks in patients with some types of aortic mechanical valve where we can anticipate haemodynamic instability when establishing an arteriovenous loop. Transapical access may also be appropriate in patients when the procedure via transfemoral access was unsuccessful.
Transapical access requires general anaesthesia, in order to perform a mini-thoracotomy allowing direct exposure of the left ventricular apex. It is rarely performed by direct apical puncture. To define the best place for incision or puncture it is advisable to do a CT scan and coronary angiography to avoid damage to the coronary arteries.
When the left ventricular apex is punctured directly, a 0.018” wire is introduced through the needle and a 5-6 Fr introducer is placed in the LV. With the help of a Judkins right or a multipurpose catheter and a hydrophilic wire, the leak is crossed and the wire is exchanged for a high support 0.035” wire. Occasionally, it is necessary to establish an AV loop (transseptal-transapical) to advance the sheath through the leak when the defect is very calcified.
When the device has been advanced through the sheath, the stability of the device has been tested, the prosthesis discs are found to be functioning well and the flow through the leak has disappeared or decreased sufficiently, the device is released.
After the device is deployed, the introducer is removed and the access is closed surgically or with a duct occluder or collagen device if the access was by direct puncture.
TRANSFEMORAL ACCESS
The femoral access is most commonly used for closure of both mitral and aortic leaks.
Aortic leaks are less frequent than mitral and in most cases can be closed directly via the retrograde approach from aorta to left ventricle. In cases of very calcified and/or tortuous leaks it might be necessary to use a transseptal approach and catch the wire passed through the leak in the left ventricle making an arteriovenous loop to facilitate the passage of the delivery sheath. Closure procedures are performed under general anaesthesia or deep sedation with fluoroscopic and 2-D or 3-D transoesophageal echocardiographic guidance.
Aortic PVLs are closed using the retrograde technique except in cases where aortic and mitral PVLs were simultaneously closed in the same procedure. In the retrograde technique, a hydrophilic wire is used to cross the defect. After the defect is crossed with the wire, the delivery sheath is advanced into the LV and the closure device is finally deployed in standard fashion. In patients with simultaneous aortic and mitral PVLs, the retrograde approach is used to cross both, with a hydrophilic wire snared in the left atrium and exteriorised through the femoral vein, establishing an arteriovenous (AV) loop. The delivery sheath is advanced anterogradely over the AV loop from LA to LV and to aorta (Ao), and both leaks are closed deploying the first device in the aortic PVL, and then the second device in the mitral PVL, consecutively through the same sheath (Figure 2).
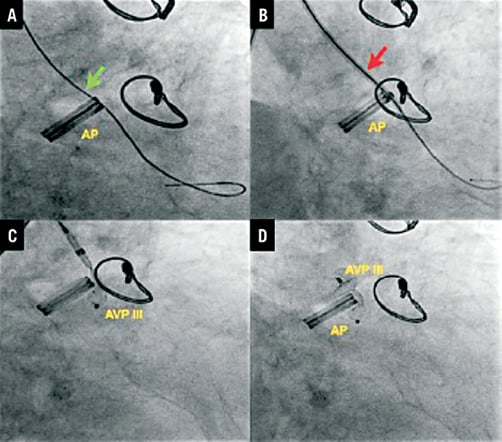
Figure 2. Aortic leak closure. A) Terumo wire through aortic leak (green arrow). B) Amplatzer delivery sheath (red arrow). C) Deploying AVP III device. D) Device release (distal disc into left ventricle and proximal disc into the aortic root). AP: aortic prosthesis
Mitral PVLs are closed using the anterograde or the retrograde approach. In the anterograde approach, after transseptal puncture, the defect is crossed with a wire into the left ventricle. In most cases the wire is further advanced into the ascending aorta and an AV loop is formed using a gooseneck snare in the ascending aorta previously introduced from the femoral artery. The delivery sheath is advanced over the AV loop from the venous aspect to the LV through the leak, and the closure device is deployed in standard fashion. In the retrograde approach for mitral PVL closure, the defect is crossed with a wire from LV to the LA, and an AV loop is established. The delivery sheath is advanced over the AV loop from the LA to the LV through the leak, and the closure device is deployed in standard fashion (Figure 3).
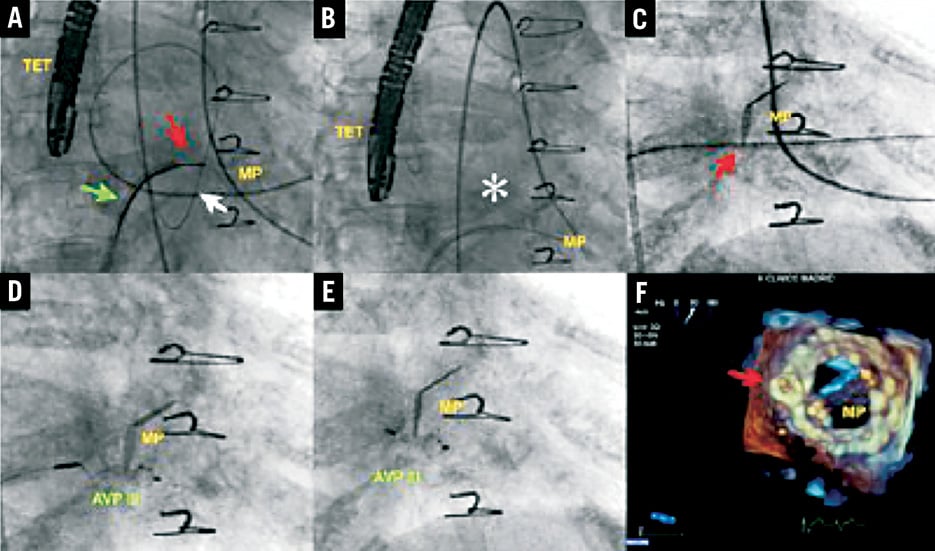
Figure 3. Mitral leak closure. A) Arteriovenous loop: transseptal catheter through interatrial septum (green arrow), hydrophilic wire passing retrogradely through leak from left ventricle (white arrow), Amplatz Gooseneck catheter (red arrow) snaring wire in left atrium. B) Arteriovenous loop (asterisk) established. C) Delivery sheath passing through mitral leak (red arrow). D) AVP III device being deployed. E) AVP III released (distal disc into left ventricle and proximal disc into the left atrium). F) 3-D echocardiography showing final result with AVP III closing the mitral leak (red arrow). MP: mitral prosthesis; TET: transoesophageal transducer
SPECIAL TIPS AND TRICKS
1) “Swan-Ganz balloon anchoring”. In cases where a transseptal puncture cannot be performed, the deployment of the device is done entirely retrogradely using a Swan-Ganz balloon anchoring manoeuvre. After crossing the mitral PVL from the LV with an hydrophilic wire, an end-hole Swan-Ganz catheter is advanced into the LA, and the balloon is inflated (anchored) in the atrial aspect of the PVL, enabling the exchange of the hydrophilic wire for a high support wire, which facilitates the crossing of the PVL with a sheathless hydrophilic guiding catheter that allows device deployment (Figure 4).
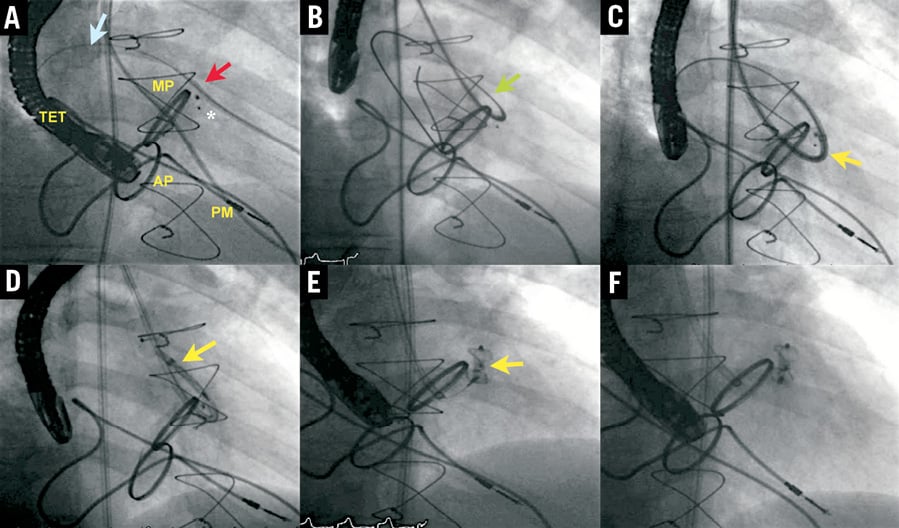
Figure 4. Mitral leak closure using Berman catheter. A) Berman catheter passing through anterolateral mitral leak (red arrow) over Terumo wire (blue arrow); previous AVP III device (asterisk). B) Extra-Stiff Wire using Berman balloon to anchor (green arrow). C) Amplatzer delivery system passing through the mitral leak (yellow arrow). D and E) Deploying AVP III device (yellow arrows). F) Final result. MP: mitral prosthesis; AP: aortic prosthesis; PM: pacemaker; TET: transoesophageal transducer
2) “Veno-venous loop”. In patients with mitral PVLs and specific types of aortic mechanical prostheses which produce haemodynamic instability, an innovative approach must be conceived. An hydrophilic catheter is advanced anterogradely through the mitral prosthesis, crossing the mitral PVL from the LV retrogradely. The wire is then snared in the LA, exteriorising it through the femoral vein to establish a veno-venous loop to allow delivery of the device.
3) “Anterograde-retrograde cross”. In patients with two mitral leaks in different locations (e.g., anterolateral and posteroseptal), the anterolateral PVL is crossed anterogradely and the same wire is used to cross the posterolateral PVL retrogradely, snaring it at the LA with a second transseptal sheath to allow the deployment of both devices (Figure 5).
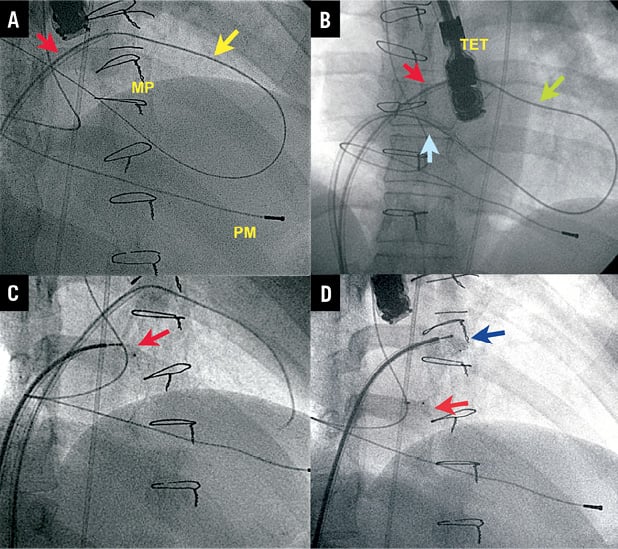
Figure 5. Mitral leak closure using a veno-venous loop with dual transseptal puncture. A) First transseptal puncture with Mullins sheath (red arrow); straight Terumo wire through anterolateral leak by anterograde approach and through posterolateral leak passing retrogradely. B) Second sheath (blue arrow) after snaring the Terumo wire, establishing a veno-venous loop. C) Device delivered in posterolateral leak (red arrow). D) Second device delivered in anterolateral leak (blue arrow). MP: mitral prosthesis; PM: pacemaker; TET: transoesophageal transducer
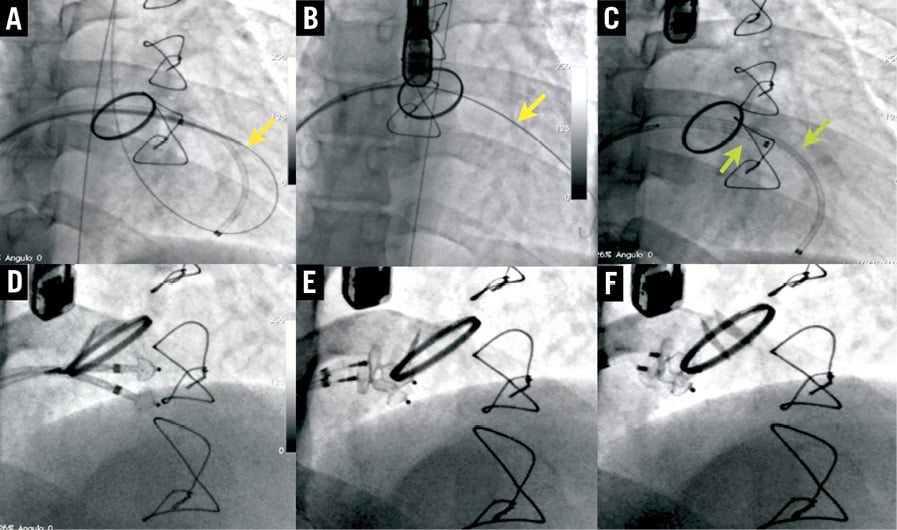
Figure 6. “Two devices at once” mitral leak closure. A and B) Sheath advanced through AV loop, dilator withdrawn and high support wire (yellow arrow) introduced in LV. C) Two sheaths (green arrows) introduced through both wires. D, E and F) Two devices deployed simultaneously.
4) “Two devices at once”. In patients with very large defects, the leak is first crossed retrogradely and an arteriovenous loop is established, positioning the first sheath in the LV directed to the apex. Through this sheath a high support wire is introduced in the left ventricle and two different sheaths are advanced, allowing the deployment of two devices simultaneously (Figure 6).
Figure 7. Several devices used in the early PVL closure experiences.
DEVICES
There is no specific device specially designed to repair paravalvular leaks. The first procedures were performed using the Rashkind double-umbrella device with total or partial success in three of the four patients in whom closure was attempted. Subsequently, various devices from the Amplatzer family (AGA Medical, Plymouth, MN, USA) have been used. These devices were designed for closure of atrial septal defects (ASD), ventricular septal defects (VSD) and patent ductus arteriosus (ADO) and, occasionally, vascular coils have been used. Lately, Amplatzer Vascular Plugs II (AVP II) and III (AVP III) are considered to be the most adequate devices to close these defects. With the use of these multiple devices, clinical results are sometimes difficult to interpret because of the different definition the various authors give to clinical success (Figure 7, Figure 8 and Figure 9)10,12,51,52.
Figure 8. Amplatzer Vascular Plug II.
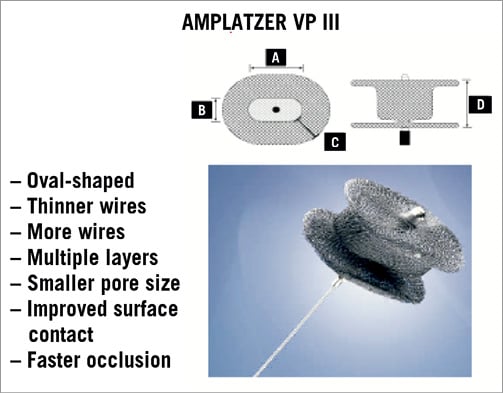
Figure 9. Amplatzer Vascular Plug III.
Results and prognosis
MEDICAL TREATMENT
Rallidis in his article “Natural history of early aortic paraprosthetic regurgitation: a five-year follow-up” concludes that “paraprosthetic aortic leaks detected early after surgery, in the absence of valve infection, are common, usually small, and have a benign course”. However, the development of new, usually severe, regurgitation associated with heart failure and haemolytic anaemia increases mortality and morbidity.
With respect to the severity of aortic PVLs in the paper by Sponga, survival was negatively affected both by commonly identified comorbidities (diabetes, stroke, pulmonary disease, renal failure, peripheral vascular disease) and also by the presence of more than grade 1 residual aortic regurgitation which was an independent predictor of postoperative mortality24.
Jindani reported that the presenting features of PVLs of the mitral prosthetic valve at 15-year follow-up included cardiac failure in 72%, bacterial endocarditis in 12% and haemolytic anaemia in 12%.
SURGERY
Surgery is recommended in patients with symptomatic leaks, but the operative mortality is high (6-42%) and increases significantly with the number of previous operations, prior myocardial infarction, diabetes mellitus, chronic obstructive pulmonary disease, NYHA Class III/IV, pulmonary artery pressure >60 mmHg, active infective endocarditis, renal insufficiency and more than one repeat valve replacement, with hospital mortality of 12.6%, 14.9% and 37% after the first, second and third or subsequent operation46,47,50,56.
These interventions also have a high rate of morbidity: perioperative stroke 5.1%, wound infections and reconstruction 25%, prolonged hospital stay >14 days 29%, infective endocarditis 7.5%, and recurrent leak in the same area in 20-37%44-50. At follow-up, new valve replacement is required in 40-68% of cases44,49. Late mortality is high with 10 and 18-year survival of 63%48 and 42.5%44, respectively.
PERCUTANEOUS TREATMENT
TRANSAPICAL
There are few reports concerning this technique. Swaans reported seven patients with aortic and mitral PVLs. The procedure was technically successful in all, with significant reduction of regurgitation in 42%. A postprocedural complication was haemotothorax, requiring surgery in 14.2% of patients. Median hospitalisation duration after the procedure ranged from 5 to 59 days57. Thourani reported three patients with mitral PVL using Amplatzer closure devices (AGA Medical Corp, Plymouth, MN, USA). All three patients had decreased regurgitation at the site of the closure as well as symptomatic improvement in their heart failure58. Ruiz reported on the transapical approach in 32 patients with 38 mitral PVLs. He used different devices with success in 89% of the cases. He reported the following complications: two patients had acute embolisation of the device, and one patient had electromechanical dissociation and death five days after the procedure. Eighteen patients improved by 1 NYHA functional Class, and seven patients did not. Sorajja used the transapical approach in 13 patients, and reported 30.6% haemothorax and 7.6% sepsis and empyema.
TRANSFEMORAL
The technique was first described in 1992 by Hourihan52 using a double-umbrella device (Rashkind or Clamshell). Subsequently, Eisenhauer8 described a technique using a Gianturco-Grifka device for mitral periprosthetic closure, and Phillips9 reported percutaneous closure of an aortic defect with an Amplatzer device. In the first series of Hein60 and Pate61-63, reported success rates were around 50%. Adverse events associated with the procedure included inability to cross the leak with the catheter wire or sheath, interference with the disc function of the prosthesis, residual leak, haemolysis and endocarditis.
After these initial experiences, the cumulative experience of the operators, the use of hydrophilic catheters, 3-D TEE guidance and the use of the various devices have improved the results (Table 1 and Table 2). Sorajja10,11 described 115 patients in whom various devices were used: ASD, ADO, VSD or Vascular Plug II. Transfemoral access was predominantly used, but in 13 patients the transapical approach was used. The reported success rate was 77%. There was no procedure-related mortality. At 30-day follow-up, the complication rate was 8.7% (1.7% sudden death, 2.6% stroke, 0.9% emergency surgery for disc impingement, 5.2% bleeding, 0.8% vascular complications, 2.6% elective surgery for unsuccessful repair). At three-year follow-up Sorajja11 reported a survival rate of 64.3%.
Garcia’s experience includes 166 patients with different devices (mainly ADO), and a few cases with ASD, VSD and vascular plugs65. Since 2008 we have used exclusively the Amplatzer Vascular Plug III device. We attempted closure via transfemoral access in 66 patients (54 with mitral and 12 with aortic leaks)66. In the 54 patients with mitral leaks, we attempted to close 60 PVLs in 63 procedures. Procedural success was 96.2% per patient. Immediately after the procedure, mitral PVL regurgitation decreased an average of 1.3±0.65 grades. At six-month follow-up, improvement of NYHA functional Class of at least 1 grade occurred in 80% (p=0.03). Compared with baseline, haematocrit improved significantly.
In 12 patients, repair of 13 aortic PVLs was attempted. The procedure was successful in 91% of the cases and aortic regurgitation decreased by 1±0.8.grades. At two-year follow-up, 91% had improvement of functional class. Late clinical events included new intervention for moderate to severe residual regurgitation through the leak in two patients (one surgical, one percutaneous at 246 and 727 days after the procedure, respectively). One patient died 831 days after the procedure from severe, untreatable coronary artery disease64.
Discussion
PVL is a well-known complication following surgical valve replacement with a reported incidence of major PVLs of 1-5%, which increases to 25-40% in surgical reinterventions of previous PVLs2,4. Clinically, PVLs can manifest as heart failure, haemolysis or, most commonly, both. Traditionally, surgical treatment with repair or, more frequently, new valve replacement has been the “gold standard” treatment for these patients. However, reoperation is associated with mortality which has been reported as high as 13%, 15% and 37% for the first, second and third procedures, respectively5. The percutaneous approach has been proposed as an alternative treatment to reoperation.
We believe that the cumulative experience of the operators, the use of hydrophilic catheters, 3-D echocardiographic guidance and the use of more user-friendly devices contribute to the high technical success in the last reported series.
The use of 3-D TEE during intervention can assist the operator in crossing the defect, thus reducing the risk of intervention failure. It can prevent complications such as interference with valve leaflets, it can assist in device size selection and it can help when more than one device is needed to close the defect. Three-dimensional TEE locates the defect precisely, gives accurate measurements and shape, guides the deployment and assesses the result.
Currently, there are no devices available that are specifically designed for PVL closure. The Amplatzer Vascular Plugs II & III were designed to embolise blood vessels. However, due to their design and mechanical characteristics, they have recently been used for transcatheter closure of PVLs. We believe that the design of the devices has improved stability without increasing the device profile.
One limitation of this technique is a steep learning curve, especially in mitral PVLs. The procedure is complex, time-consuming, and not always successful. The approach to the aortic leaks is usually straightforward. In cases of mitral leaks a systematic approach can be designed, but the variability of the anatomy forces the operator to introduce different variations to the initial plan often during the procedures.
With regard to the safety of the procedure, the rate of severe complications is low, considering the severity of the disease treated and the often poor clinical condition of the patients.
Conflict of interest statement
The authors have no conflict of interest to declare.

