Paravalvular leaks (PVL) represent pathological communications between the native annulus and the prosthetic valve, develop more frequently in the mitral than in the aortic position, and more often in mechanical than in biological prostheses. They are typically manifested by heart failure and haemolytic anaemia.
The diversity of PVL warrants an individualised treatment strategy according to surgical risk and anatomic suitability for transcatheter closure (Figure 1). Current guidelines recommend redo surgery for clinically relevant PVL as the preferred treatment strategy. Transcatheter PVL closure has been established as an alternative to surgery in inoperable or surgical high-risk patients with suitable anatomy1,2. The complexity of transcatheter PVL closure requires the combined expertise of multimodality imaging specialists, cardiac surgeons and structural interventionalists. While a random-effects meta-analysis of five observational studies suggested comparable rates of mortality in patients undergoing surgical and transcatheter PVL closure, redo surgery and transcatheter intervention may be complementary rather than competing strategies in many instances3.
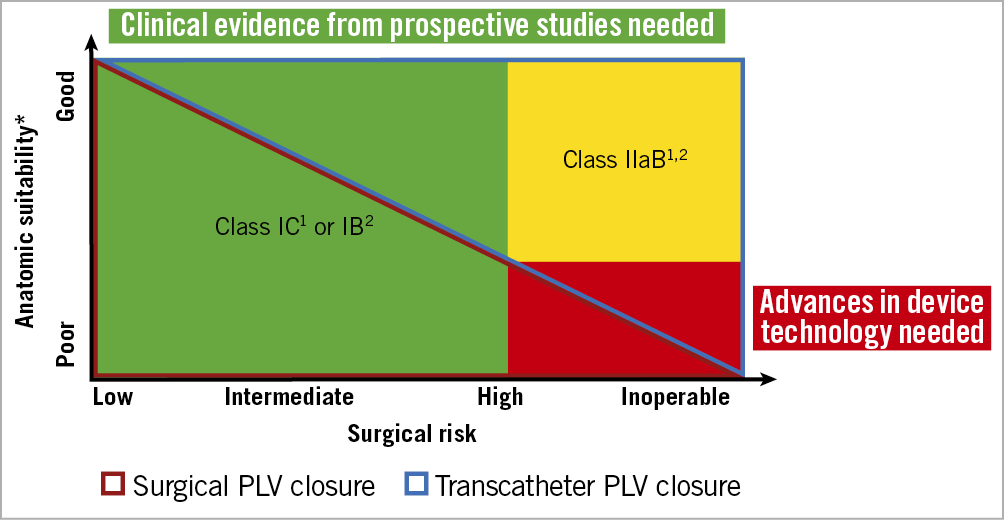
Figure 1. Treatment recommendations for paravalvular leaks. ESC/ EACTS guidelines1 and the ACC/AHA guidelines2 for the management of patients with valvular heart disease and future perspectives. *Anatomic suitability determined by location (mitral/aortic), valve type (mechanical/bioprosthetic) number, distribution, size, extension (% of circumference) and shape (oval, crescentic, tunnel-shaped, slit-like) of paravalvular leaks (PVL).
Available evidence is limited to non-randomised studies including modest numbers of patients with PVL of different aetiologies in different anatomic locations (mitral/aortic) and valve types (mechanical/bioprosthetic), differences in the distribution, size, extension (percent of circumference) and shape (oval, crescentic, tunnel shaped, slit-like) of PVL, and varying endpoint definitions4,5,6. Underreporting of rates of clinical success (defined by the reduction of haemolysis and improvement of heart failure) as opposed to technical (device implantation) and procedural (PVL reduction) success underscores the need for prospective studies with thorough clinical and echocardiographic follow-up7.
In the current issue of the Journal, Perl and colleagues8 report long-term outcomes (mean follow-up of 5.6±6.1 years) of transcatheter PVL closure in intermediate- to high-risk patients at a single tertiary care centre. In a cohort of 100 procedures (74 for mitral PVL and 26 for aortic PVL) in 95 patients, the device was successfully implanted in 88 cases (88.0%). Among 77 procedures for which echocardiographic follow-up was available at 1.5 to 2.0 years, no more than mild PVL was documented in 59 procedures (76.6%). Mortality rates were 3.8% at 90 days, 15.6% at one year, and 27.2% at five years. The authors report a sustainable improvement in New York Heart Association (NYHA) functional class at three years (NYHA III or IV: 97.6% to 34.5%), as well as recovery from haemolysis and anaemia, as evidenced by the reduction of lactate dehydrogenase (LDH) levels, reduced need of blood transfusions and increase in haemoglobin levels.
The study reports long-term clinical follow-up of patients after PVL closure and supports the safety and effectiveness of transcatheter intervention in selected patients as an alternative to surgery. While the study provides a detailed account of procedural data and delineates the potential of transcatheter PVL closure at an experienced centre, the reported findings need to be interpreted with caution. The exclusion of patients undergoing surgical PVL closure or conservative management introduces a selection bias; at the same time, incomplete echocardiographic follow-up compromises the reliability of the presented data. A detailed description of the anatomic characteristics of patients referred to surgery on one hand and of patients with transcatheter procedural failure on the other may prove helpful in the refinement of a valid treatment algorithm for these patients, and could be addressed in a future analysis.
The landscape of valve therapy is in transition. As the field is gravitating towards transcatheter valve replacement, the pathomechanisms of PVL are subject to change and new challenges are about to emerge. The remaining native leaflets and the stent frame may complicate plug-based PVL closure3. Conversely, transcatheter heart valves allow for novel approaches of PVL closure such as post-dilatation and valve-in-valve implantation. While moving targets are the hardest to hit, the emerging challenges in this envolving field may accelerate progress and close current gaps of knowledge.
Conflict of interest statement
T. Pilgrim reports research grants to the institution from Edwards Lifesciences, Boston Scientifc and Biotronik, personal fees from Biotronik and Boston Scientific, participation in a clinical event adjudication committee for a study sponsored by HighLife SAS and reimbursement of travel expenses from Medira. He is a proctor for Medtronic. T. Okuno reports speaker fees from Abbott.
Supplementary data
To read the full content of this article, please download the PDF.

