Abstract
Background: In most centres, clinically significant percutaneous paravalvular leak (PVL) closure following valve replacement surgery is reserved for those considered high-risk for surgery. There is a paucity of data regarding the long-term outcomes of these patients.
Aims: Our goals were to assess the long-term outcomes of patients undergoing percutaneous PVL closure.
Methods: A total of 100 consecutive transcatheter PVL closure procedures (74 mitral, 26 aortic) were performed in 95 patients between February 2005 and August 2019 at our hospital. Data collected included procedural success rates, indication-specific outcomes and mortality.
Results: Mean follow-up was 5.6±6.1 years, mean age 62.6±15.2 years, and 45.4% were female. The device was successfully implanted in 88 procedures (88.0%). Patients who presented with heart failure (n=57) had a significant improvement in NYHA classification (29.2% Class III/IV versus 100.0%, p<0.001). For patients who presented with haemolytic anaemia (n=38), haemoglobin increased (11.94±1.634 vs 9.72±1.49, p<0.001) and LDH levels were reduced (1,354.90±1,225.55 vs 2,039.40±1,347.20, p<0.001) following the procedure. Rates of mortality were 3.8% at 90 days, 15.6% after 1 year, and 27.2% after 5 years.
Conclusions: For patients who are deemed intermediate- to high-risk for repeat surgery, transcatheter PVL closure shows reasonable clinical success rates, with a significant improvement in symptoms, and a relatively low rate of periprocedural complications.
Introduction
Following heart valve surgery, 2-18% of patients will ultimately develop significant paravalvular leak (PVL)1,2,3,4,5,6. It occurs more commonly following mitral valve replacement (MVR) than after aortic valve replacement (AVR) and following mechanical rather than biological prosthetic valve implantation2. The occurrence of PVL is also increased following incident infective endocarditis, and is related to a supra-annular versus an annular aortic prosthesis, suturing technique, extensive annular calcification and tissue friability2,7,8.
Most PVLs occur shortly after surgery, are usually small and may close spontaneously due to epithelialisation. However, the development of a new valve regurgitation should raise suspicion of PVL. In addition, prosthetic valve endocarditis or a prosthetic valve failure must be ruled out. While some go undetected, 2-5% of all valvular surgery patients are expected to suffer from symptoms related to it. The main signs and symptoms associated with significant PVL are dyspnoea, heart failure (HF) and/or left ventricular enlargement, haemolysis and symptomatic anaemia. Importantly, PVL rates continue to increase over time, with significant increase in rates after 10 and 20 years9. Nevertheless, there is a paucity of data regarding the long-term natural history of patients with PVL1,2,3,4,5,6 , as well as following percutaneous treatment of PVL10,11,12,13.
Recent guidelines recommend repeat operation if PVL is related to endocarditis or causes significant haemolytic anaemia (HA), requiring repeated blood transfusions or leading to severe symptoms (class I, level of evidence C), whereas percutaneous PVL closure may be considered for high-risk cases only (class IIb, level of evidence C)14. However, a recent single-centre study examined the long-term outcomes of patients with PVL after mitral valve and aortic valve surgery who were considered eligible for a reoperation. Most underwent surgery, yet the outcomes for both the short- and long-term mortality were relatively poor (3% in-hospital mortality and 47% mortality at 6.6±4 years)15. In general, supportive data for either the surgical or the percutaneous approach to PVL repair are relatively scarce. We thus aimed to assess the long-term outcomes (up to five years) of percutaneous PVL closure at our tertiary centre, focusing on outcomes of mitral versus aortic PVL.
Methods
PATIENTS AND SETTING
This observational study is based on a prospective registry of all-comers for percutaneous PVL closure procedures, derived from the Rabin Medical Center, Israel, between February 2005 and August 2019. All patients suffered from symptomatic PVL following mitral valve or aortic valve surgery. Patients who were not deemed suitable for percutaneous PVL closure were those with suspected or proven bacterial endocarditis and those with very large leaks, encompassing more than one quarter of the valve perimeter. Most of these patients had reoperation and are not included in our study.
Patient data, cardiovascular risk factors, and procedural dates were entered into a database at the time of the procedure. All patients had a comprehensive transthoracic echocardiogram (TTE) and transoesophageal echocardiogram (TEE) at the time of initial assessment. Echocardiographic data at 1.5-2.0 years were available for 77 procedures (77.0%) performed in 74 (77.9%) patients, enabling us to determine the degree of residual leak.
Ethics approval was granted for this study by the institutional review board.
PROCEDURE
All procedures were performed with the informed consent of the patient, following Heart Team discussion. The procedures were carried out under general anaesthesia for all mitral PVLs and most aortic PVLs using fluoroscopic and TEE guidance. In some of the aortic PVLs, the procedure was performed in conscious patients, using intra-cardiac echo (ICE) (n=7) or TTE (n=1) guidance. A routine TTE was performed in all patients on the following day. The different techniques, approaches and devices used for PVL closure have been described extensively before and are beyond the scope of this paper. However, some principles of PVL closure that we adopted are outlined in Supplementary Appendix 1.
OUTCOMES
The date of percutaneous PVL closure at our institution was defined as the beginning of the observational period. Periprocedural outcomes were recorded. Immediate and in-hospital clinical events were prospectively recorded in the institutional database. During follow-up, patients completed standardised questionnaires on clinical events at six-month intervals either by telephone or in the outpatient clinic. When indicated, records from peripheral hospitals were acquired to verify the events. All events were further confirmed and adjudicated by the institutional clinical events adjudication committees. Survival status, as well as cause of death, in case it had occurred, was ascertained from municipal civil registries at one and five years.
Clinically significant haemolytic anaemia was defined as symptomatic anaemia (haemoglobin <13 g/dl in women or <15 g/dl in men) requiring transfusion, with laboratory evidence of intravascular haemolysis. HF was diagnosed by our HF team and was assessed according to the New York Heart Association (NYHA) classification. Renal failure was defined as glomerular filtration rate below 50 mL/min/1.73 m2, calculated according to the Modification of Diet in Renal Disease formula.
The degree of PVL was defined as mild, mild to moderate, moderate, moderate to severe or severe. Procedures were defined as successful, partially successful, or unsuccessful – determined by whether any echocardiographic visible leakage was observed following the procedure. An unsuccessful procedure was diagnosed in cases of no change in the leakage observed directly after the procedure or failure to insert the device. Partial success was defined as showing some improvement in the leakage, or when not all leaks were taken care of in patients with more than one leak around the same valve. Success was affirmed in cases with no more than mild residual leak. For those patients with available echocardiographic information at long-term follow-up, we assessed the degree of PVL after 1.5-2.0 years.
Comparison was made between patients with PVL following AVR versus MVR, as well as according to the main indication for intervention – HF or anaemia. Primary endpoints included mortality at 90 days, 1 year and 5 years. Secondary endpoints comprised procedural success rates, rates of procedural complications, and improvement in NYHA class (for those with predominant HF), haemoglobin, lactate dehydrogenase (LDH) and number of packed red blood cell units (PC) transfused (for those with predominant anaemia).
STATISTICAL ANALYSIS
Demographic, procedural and outcome data were divided according to the valve treated – following AVR or MVR. Continuous data are summarised as mean and standard deviation, or median and interquartile range (IQR), and categorical data as frequency (%). The Student’s t-test or analysis of variance was used to compare continuous variables between groups, and the ANOVA test was used for categorical variables. The normality of variable distributions was assessed using the Kolmogorov-Smirnov test. Time-to-event curves were constructed using the Kaplan-Meier method and compared using the log-rank test. Effect sizes are presented as odds ratio and 95% confidence interval (CI). All statistical analyses were performed with SPSS, Version 26 (IBM Corp., Armonk, NY, USA). A p-value of <0.05 was considered statistically significant.
Results
One hundred procedures were performed in 95 patients. Mean age was 62.6±15.2 years, and 42.0% were female patients. The mean Society of Thoracic Surgeons (STS) score was 7.5±5.5. Seventy-four (74) cases (74.0%) were performed for mitral PVL, 26 (26.0%) for aortic PVL. Of these procedures, 78 (78.0%) were performed as the initial correction of PVL, 12 (12.0%) were carried out after one surgical PVL correction and 10 (10.0%) were performed following two repeat surgeries (Table 1). Mitral PVL, as compared to aortic PVL cases, had a higher percentage of women (44.3 vs 37.5%, p=0.046), higher values of LDH at baseline (1,778.3±1,241.8 vs 1,102.2±865.9, p=0.04) (Table 1) but lower rates of reduced left ventricular ejection fraction (p=0.03) (Table 2). Eighty-six percent (86.0%) of the patients suffered from HF prior to the procedure (84.6% for mitral and 89.8% for aortic PVL), and 41.0% from HA (45.7 vs 35.7%, p=0.094 for presenting symptoms) (Table 1).
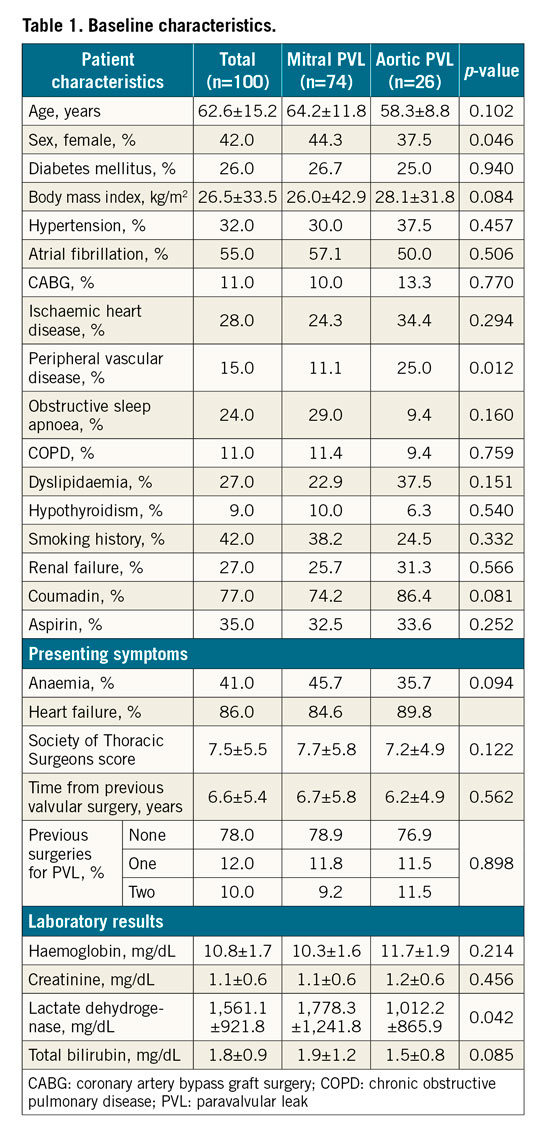
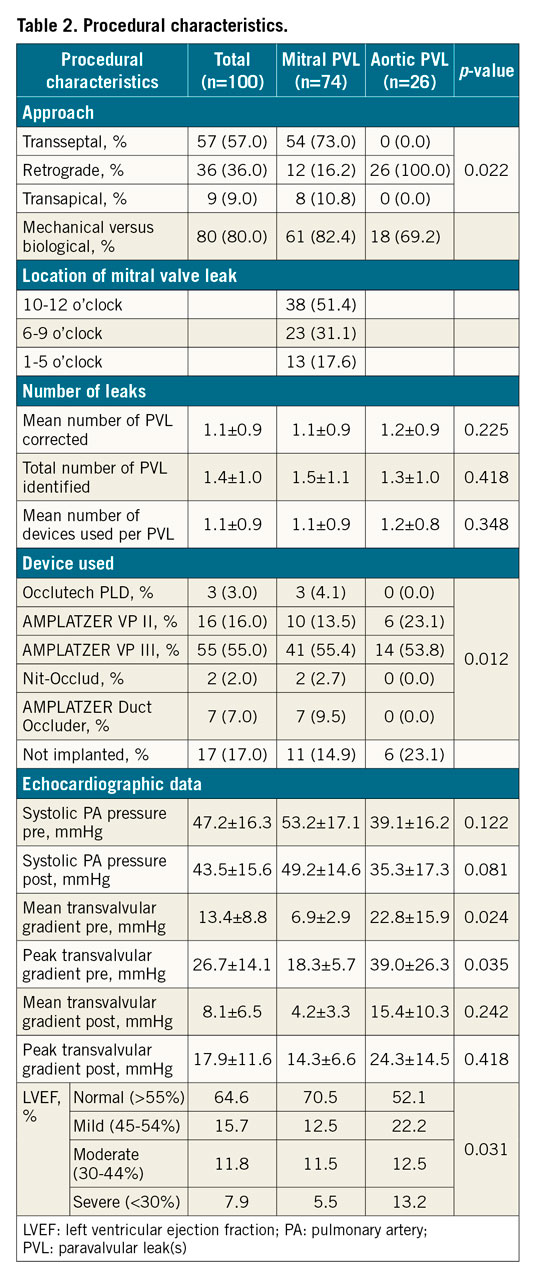
The device was successfully implanted in 88 procedures (88.0% for the complete cohort, 88.5% for aortic PVL, 77.0% for mitral PVL) (Figure 1). The transseptal approach was the most common one for mitral PVL (73.0%), whereas the retrograde approach was the sole approach in cases of aortic PVL. The transapical approach was the least common method (9.0% of all cases) (Table 2). The most common location for mitral PVL was 10-12 o’clock (51.4% of cases) (Central illustration). The most common device used (55.0% of all cases) was the AMPLATZER™ Vascular Plug III (St. Jude Medical, St. Paul, MN, USA). There were no major differences in the number of leaks identified, number treated or number of plugs per PVL between mitral and aortic PVL (Table 2).
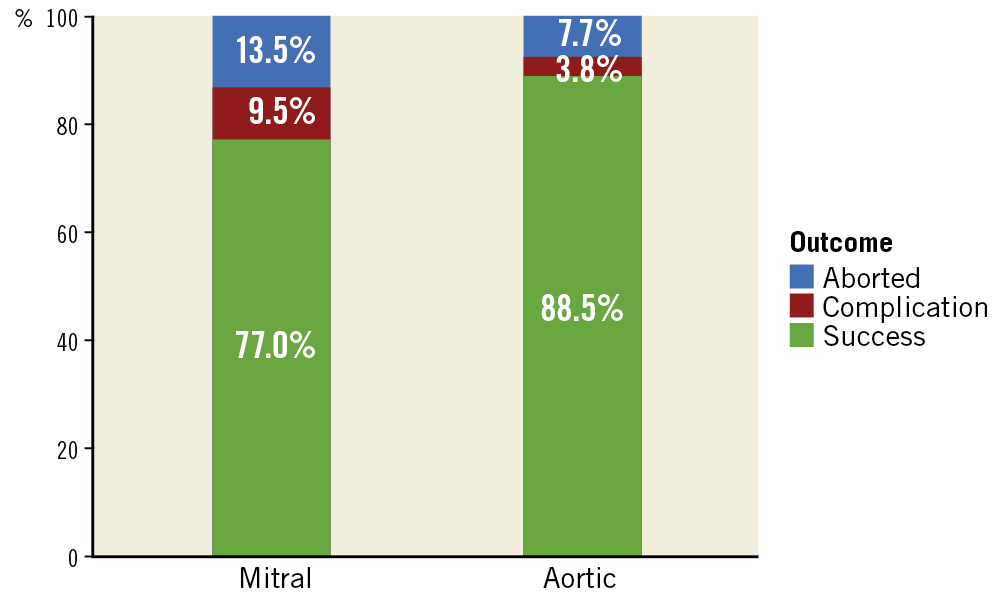
Figure 1. Outcomes by valve treated.
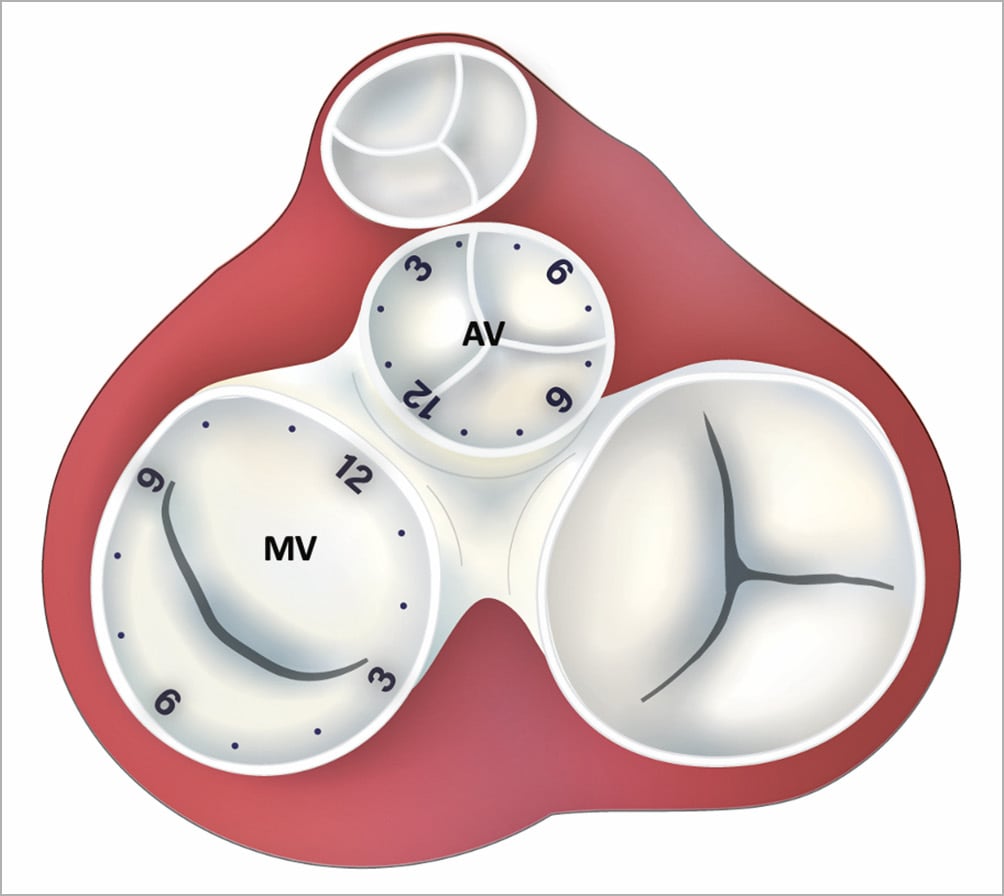
Central illustration. Anatomic location of PVL according to the clock scheme. AV: aortic valve; MV: mitral valve
The leading cause of procedural failure was the inability to cross the leak or to advance the equipment (10.0% of all cases, 11.8% for mitral, 7.7% for aortic PVL). Major periprocedural complications occurred in 8.0% of the patients (9.5% for mitral, 3.8% for aortic PVL) (Figure 1, Supplementary Appendix 2).
The median follow-up was 66 months (IQR 18-92). Rates of PVL below moderate (the definition for success at the end of the procedure) remained high – 59 of the 77 (76.6%) procedures for which we had echocardiographic information at 1.5-2.0 years were estimated as mild or below. Rates of mortality were 3.8% at 90 days, 15.6% after 1 year, and 27.2% after 5 years. Mortality was higher for patients undergoing transcatheter PVL closure of the mitral valve (log-rank p<0.01) (Figure 2) and for patients who underwent an unsuccessful transcatheter PVL closure (log-rank p<0.01) (Figure 3). NYHA class was reduced after the procedure (reduction from 97.6% NYHA III-IV at baseline to 28.6% at first follow-up for patients with mitral PVL and from 88.4% to 27.3% in aortic PVL, p<0.01 for both) (Figure 4) and after 3 years (of 55 patients with available information, NYHA III-IV was described in only 19 [34.5%]). LDH levels were reduced following the procedure (from 1,778.3±1,241.8 to 1,282.5±891.2 in patients with mitral PVL and 1,012.2±865.9 to 720.3±831.9 in patients with aortic PVL, p<0.01 for both) (Figure 5) and after 3 years (1,023.4±881.3, n=48). Levels of haemoglobin increased immediately after the procedure (from 10.3±3.9 to 11.5±4.2 in patients with mitral PVL, p=0.03, and from 11.7±8.2 to 13.0±8.6 in patients with aortic PVL, p=0.04) (Figure 6) and to 12.4±8.3 after 3 years (n=50). Of the mitral PVL patients, 37.5% required packed red blood transfusion during the year prior to the procedure versus 14.8% in the following year (p<0.001, mean number of PC received 4.8±3.8 before versus 1.2±1.0 after the procedure, p<0.001). For patients with aortic PVL, only 22.1% required PC transfusion versus 9.4% after the procedure (p=0.03), for a mean number of PC of 2.8±1.0 versus 0.7±0.8 (p<0.001). After 3 years, haemolysis had worsened in 7 patients (14.0%). These patients were all treated conservatively.
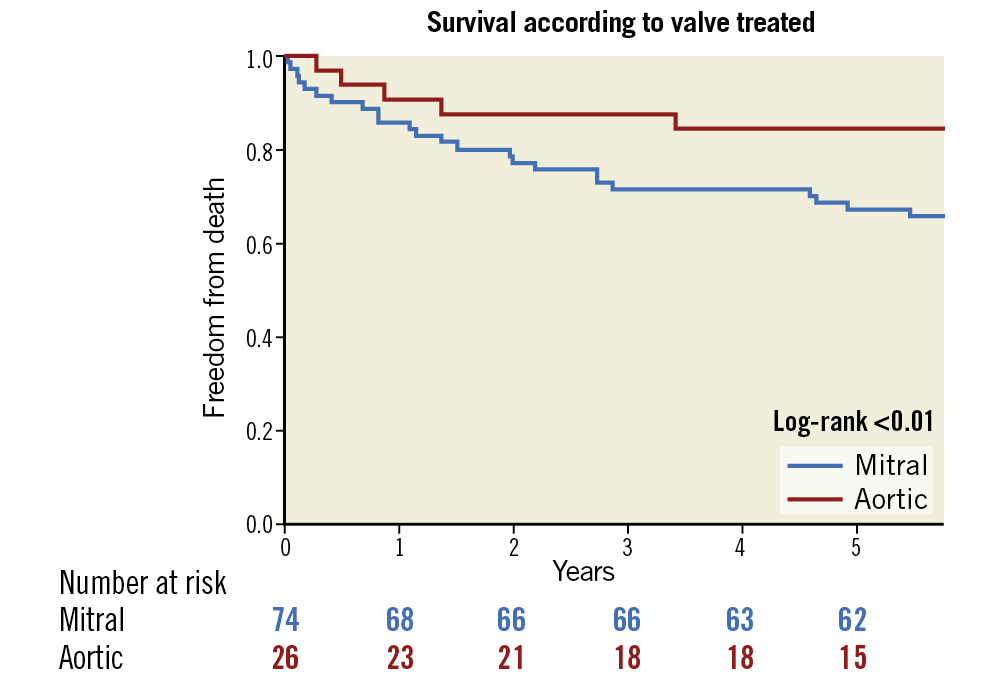
Figure 2. Kaplan-Meier survival estimates of all-cause mortality by valve treated.
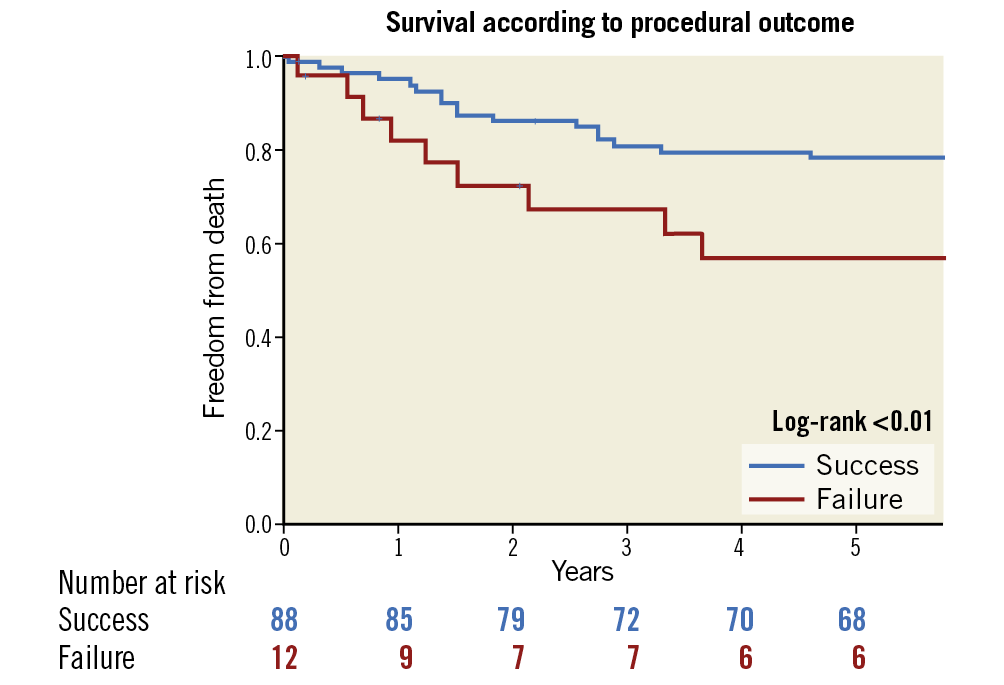
Figure 3. Kaplan-Meier survival estimates of all-cause mortality by procedural outcome.
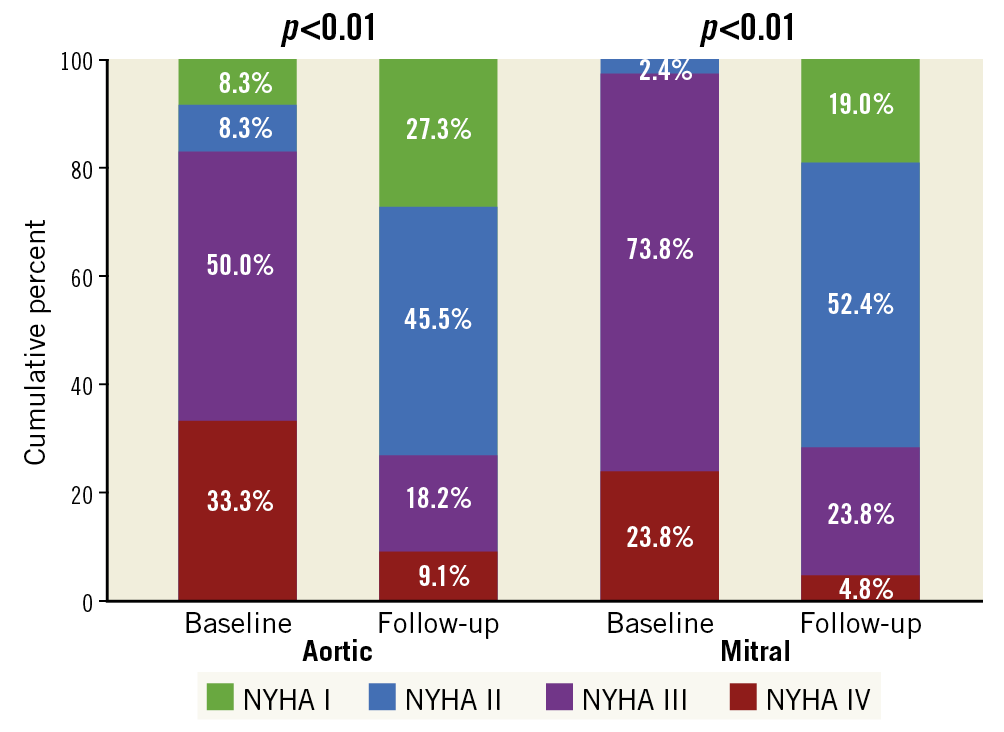
Figure 4. NYHA class by valve treated.
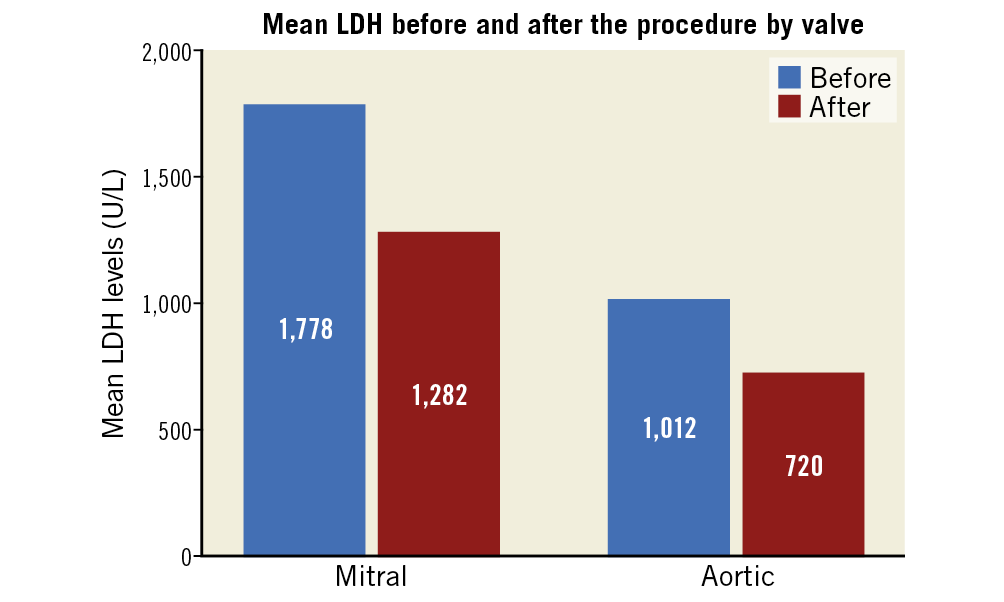
Figure 5. Mean LDH by valve treated.
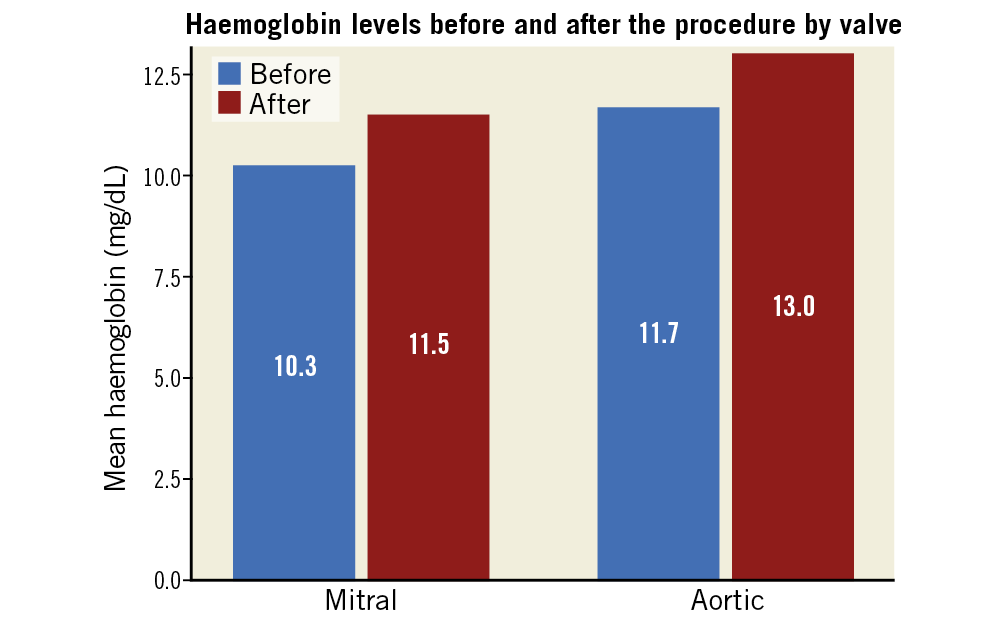
Figure 6. Mean haemoglobin by valve treated.
Patients who presented primarily with HF had significant improvement in the NYHA class (100% were Class III-IV prior to the procedure, versus 29.2% at follow-up, p<0.001). In patients who presented with HA as a main indication, there was a significant reduction in LDH level (1,354.90±1,225.55 vs 2,039.40±1,347.20, p<0.001) following the procedure, as well as an increase in haemoglobin (11.94±1.634 vs 9.72±1.49, p<0.001). A distinct group suffered from both HF and HA. In this “mixed group” (n=44), NYHA Class III-IV was reduced from 92.3% at baseline to 28.5% following the procedure (p<0.01) and LDH was reduced from 1,901.24±1,019.12 to 1,305.36±899.44, p<0.001). There was also a reduction in the number of PC received (33.7% prior to vs 13.5% post procedure, p<0.001 and 4.2±2.4 mean number of PC prior to vs 1.9±1.3 after the procedure, p<0.001).
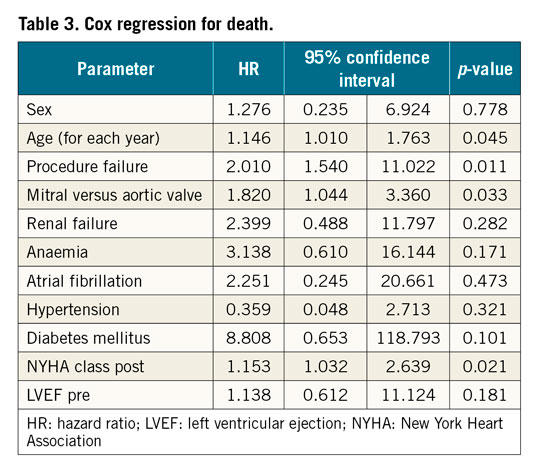
Cox regression identified four factors significantly impacting on mortality: age (HR 1.15 for each additional year, 95% CI: 1.01-1.76, p=0.045), mitral versus aortic valve (HR 1.82, 95% CI: 1.04-3.36, p=0.033), procedure failure (HR 2.01, 95% CI: 1.54-11.02, p=0.011) and the NYHA class following the procedure (HR 1.15 for each class increase, 95% CI: 1.03-2.64, p=0.021) (Table 3). There was no difference in outcomes according to the surgical risk score, type of device or the anatomical approach. Finally, the association between residual leak (more than mild) at 1.5-2.0 years and mortality at 5 years was assessed using an univariate analysis, demonstrating strong relationship (HR 2.76, 95% CI: 1.33-7.28).
Discussion
In this current work, we assessed the long-term outcomes of patients undergoing percutaneous PVL closure at our medical institution. Our results show that rates of mortality were compar-able and perhaps better than the outcomes of most studies assessing a surgical approach to PVL closure1,3,15,16,17. We have also seen significant differences in outcomes according to the valve treated, as mitral PVL closure results in worse prognosis when compared with aortic leak closure. Long-term prognosis also depends on the age of the patient, the success of the procedure and the NYHA classification at the first follow-up. Finally, we have witnessed improved measurements of HF and HA, in particular when each parameter is assessed within the group of patients referred for the procedure for that specific indication.
Following valve replacement surgery, the presence of PVL is not infrequent and increases the risk of mortality, repeat admissions and reoperation1,2,3,4,5,6. The exact mechanism of the appearance of PVL in some patients is uncertain and likely to be multifactorial. It has been suggested that some PVLs recede in the early postoperative period, especially with MVRs, due to endothelialisation of small PVLs or suture tracts7.
According to the European Society of Cardiology guidelines, the default treatment for PVL should be repeat surgery, whereas percutaneous PVL closure is reserved for inoperable or high-risk candidates14. Therefore, outcomes for patients undergoing percutaneous PVL closure are expected to be worse than repeat surgery. However, recent studies have demonstrated less than optimal outcomes for repeat surgery for PVL. Patients at particular risk include those with a high STS score, a high right ventricular systolic pressure, PVL of an infectious aetiology, and cases where PVL surgery resulted in recurrence of the leak12,15. In addition to the immediate risk of reoperation, the less-than-optimal outcomes in surgery for PVL are related to the fact that the tissues around the leakage are often calcified and friable – increasing the risk of recurrent leaks18,19. In fact, several studies have demonstrated lower rates of complications in patients treated by percutaneous PVL closure, and some even suggested it as the treatment of choice16,17,20,21.
As for the potential benefits of percutaneous PVL closure, there are two main factors – reduction in mechanisms related to HF, and improvement in HA. One previous study22 showed a modest improvement in haemolysis marker after PVL closure. In that cohort, the benefit was significantly higher in patients with a mechanical valve. A recent study assessing the Occlutech® PLD Occluder (Occlutech GmbH, Jena, Germany) demonstrated a significant reduction of haemolysis-related blood transfusions following the procedure23. Heart failure was shown to improve in most studies assessing the effect of PVL correction12,24,25. One study also demonstrated a significant association between reduction in left atrial pressure and outcomes26, reminding us of the important impact of the haemodynamic changes associated with reduction of PVL. Interestingly, in our study, when divided by each of the two indications, we witnessed a significant improvement in these two factors – haemoglobin was raised (and LDH reduced) in patients presenting with HA, and the NYHA class had improved in patients presenting with congestive HF.
The mean STS score in our study was 7.5±5.5, somewhat higher than the risk in the other large trials of percutaneous PVL closure12,15,27, but still within the intermediate- to high-risk range. Guidelines currently advocate routine follow-up of patients with prosthetic valves, which includes blood tests for HA, as well as TTE and, if needed, TEE to detect a PVL. Reoperation is recommended if the PVL is related to endocarditis or causes HA requiring repeated blood transfusions or leading to severe symptoms. Percutaneous closure of a PVL has a class IIb recommendation, due to inconsistent evidence of efficiency14. Therefore, it is important to continue to gather long-term information from medical centres such as ours, which treat patients of varying risk profile by the percutaneous approach, demonstrating no more than modest risk throughout the five-year follow-up. Our findings, in line with previous trials, support consideration of transcatheter PVL repair in a wide range of patients, including in those who are, indeed, candidates for surgery as well.
Limitations
The study is a single-centre observational study; thus we have not compared outcomes to surgical PVL correction. Therefore, our findings cannot be generalised to centres contemplating one of the two approaches. However, since our medical centre is more inclusive in its decisions to perform percutaneous PVL closure, the outcomes presented here warrant careful consideration of this approach in PVL all-comers except for those with active infection. More head-to-head comparisons of the two options may impact on future guidelines.
Conclusions
Transcatheter PVL closure in patients with intermediate to high risk for reoperation shows reasonable clinical success rates, demonstrating significant improvement in symptoms and in haemolytic anaemia, as well as a relatively low rate of periprocedural complications. Younger patients, those who were treated for aortic PVL, who undergo a successful percutaneous PVL closure, and enjoy elevated NYHA class at the first follow-up have better outcomes. These findings need expanded validation.
|
Impact on daily practice What is known? Following valvular surgery, paravalvular leak (PVL) is not uncommon. Transcatheter PVL closure is usually reserved for intermediate to high surgical risk patients in cases of symptomatic heart failure or haemolytic anaemia. What is new? In this long-term observational prospective cohort study, we have seen reasonable outcomes and improvement in symptoms according to the indication for the procedure. Aortic PVL patients have better prognosis than mitral PVL patients. What is next? More information on the correct strategy to correct PVL in these intermediate- to high-risk patients is warranted. In addition, studies comparing the different devices used for PVL may assist in our decision to treat patients with PVL. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

