
Abstract
Aims: The aim of this study was to assess the efficacy and safety of the Occlutech Paravalvular Leak Device (PLD) for the percutaneous closure of paravalvular leaks (PVL).
Methods and results: Patients with PVL were enrolled at 21 sites from nine countries. Indications for PVL closure were heart failure and/or haemolytic anaemia. Endpoint measures were changes in PV regurgitation grade, NYHA class and requirement for haemolysis-related transfusion. One-hundred and thirty-six patients with mitral (67.6%) or aortic (32.4%) leaks were included (mean age 66.7 years, 58% male); 31% had multiple PVLs. The proportion of patients with NYHA Class III/IV decreased from 77.3% at baseline to 16.9% at latest follow-up. The proportion of patients with need for haemolysis-related blood transfusion decreased from 36.8% to 5.9% and from 8.3% to 0% for ML patients and AL patients, respectively. All-cause mortality was 7.4%. Complications included interference with valve leaflets (0.7%), transient device embolisation (percutaneously solved) (0.7%), late device embolisation (0.7%), recurrent haemolytic anaemia (2.2%), new-onset haemolytic anaemia (0.7%), valve surgery (2.2%), need for repeat closure (0.7%), complications at femoral puncture site (0.7%) and arrhythmias requiring treatment (4.4%).
Conclusions: PVL closure with the Occlutech PLD demonstrated a high success rate associated with significant clinical improvement and a relatively low rate of serious complications.
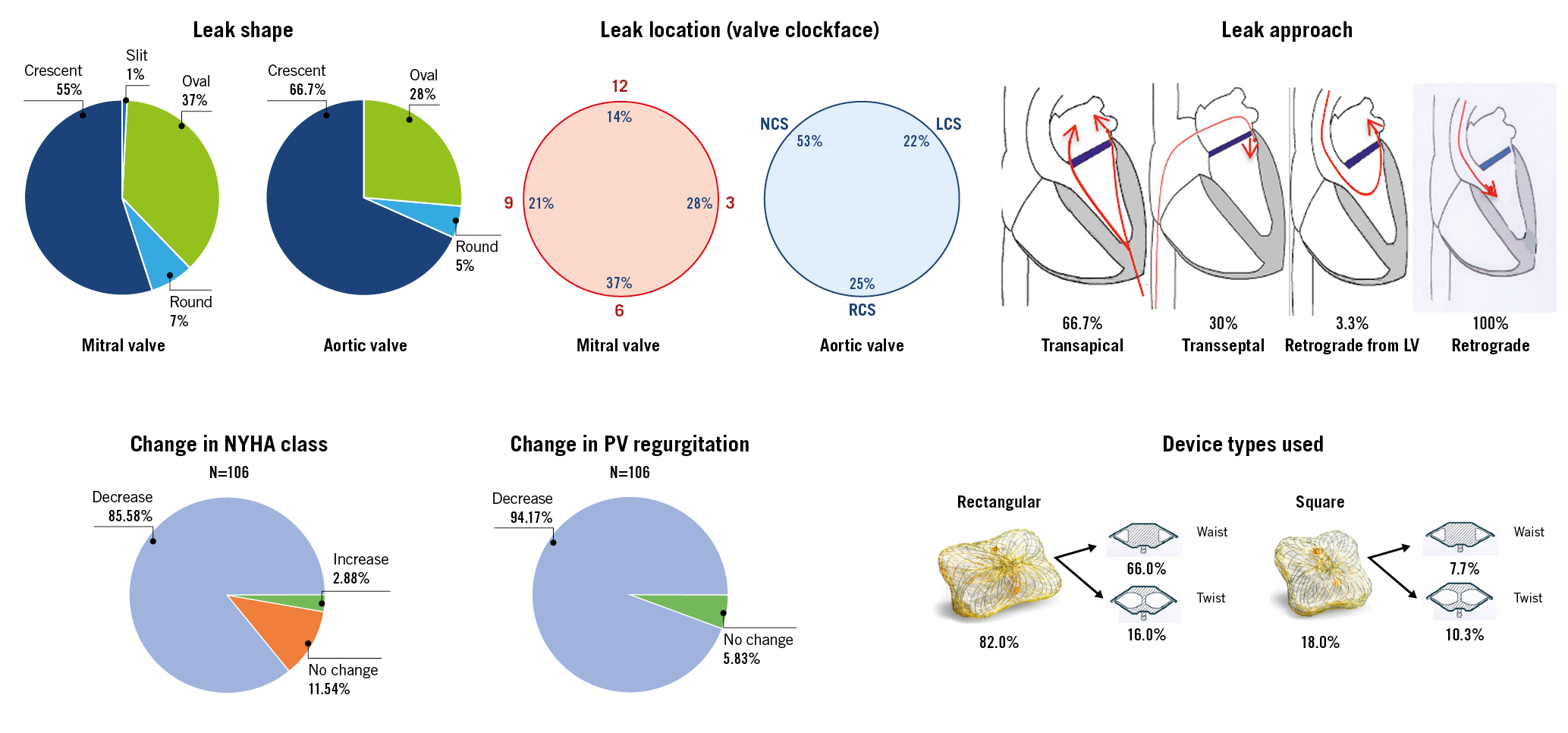
Visual summary. PVL characteristics, leak approach, device types and midterm procedural and clinical outcomes of percutaneous paravalvular leak closure with the Occlutech® PLD. Transcatheter PVL closure with the specifically designed PLD was demonstrated to be effective with a relatively low rate of major complications. Procedural success for ML and AL closure was high with a low rate of residual or recurrent leaks. Significant improvement of NYHA class, and reduction of haemolytic anaemia and transfusion dependency were achieved.
Introduction
Transcatheter closure of paravalvular leak (PVL), first described in 19921, has slowly evolved into a viable and less invasive alternative to surgery in high-risk patients with suitable anatomy2,3,4. Complete PVL sealing is relatively rare due to irregular leak morphology and the complex anatomy of the surrounding tissue. This creates the need for dedicated devices ideally available in multiple sizes and shapes for improving procedural efficacy and success. The Occlutech Paravalvular Leak Device (PLD) (Occlutech, Helsingborg, Sweden) is the only device specifically designed and certified in Europe (CE marked in 2014) for the treatment of mitral leaks (ML) and aortic leaks (AL). This international, multicentre registry was designed to provide additional clinical data on the efficacy and safety of the PLD in high-risk patients with ML or AL after surgical implantation of prosthetic heart valves.
Methods
This registry considered PLD closure procedures performed in 21 hospitals in nine EU and non-EU countries (Supplementary Appendix 1). All centres were contacted to participate in this study, and all agreed to participate. Anonymised data were acquired from medical and electronic records regarding patient medical history, demographics, vital signs, clinical laboratory tests, 12-lead electrocardiography (ECG) and transthoracic and transoesophageal echocardiography (TTE/TEE). Signed informed consent was obtained from all patients prior to the procedure. The study plan was approved by an independent ethics committee, the International Medical and Dental Ethics Commission (IMDEC).
PROCEDURE
Two- and three-dimensional (2D/3D) TEE was used throughout each procedure, particularly in ML for complete and accurate delineation of these defects (Figure 1, Figure 2, Moving image 1-Moving image 6). Three-dimensional modalities included real-time 3D zoom and full volume acquisition with and without colour flow imaging. The degree of valvular regurgitation was evaluated by Doppler echocardiography using the guidelines recommended by the American Society of Echocardiography5. In some cases, ECG-gated cardiac computed tomography (CT) angiography was employed to define the location, size, shape and trajectory of PVL.
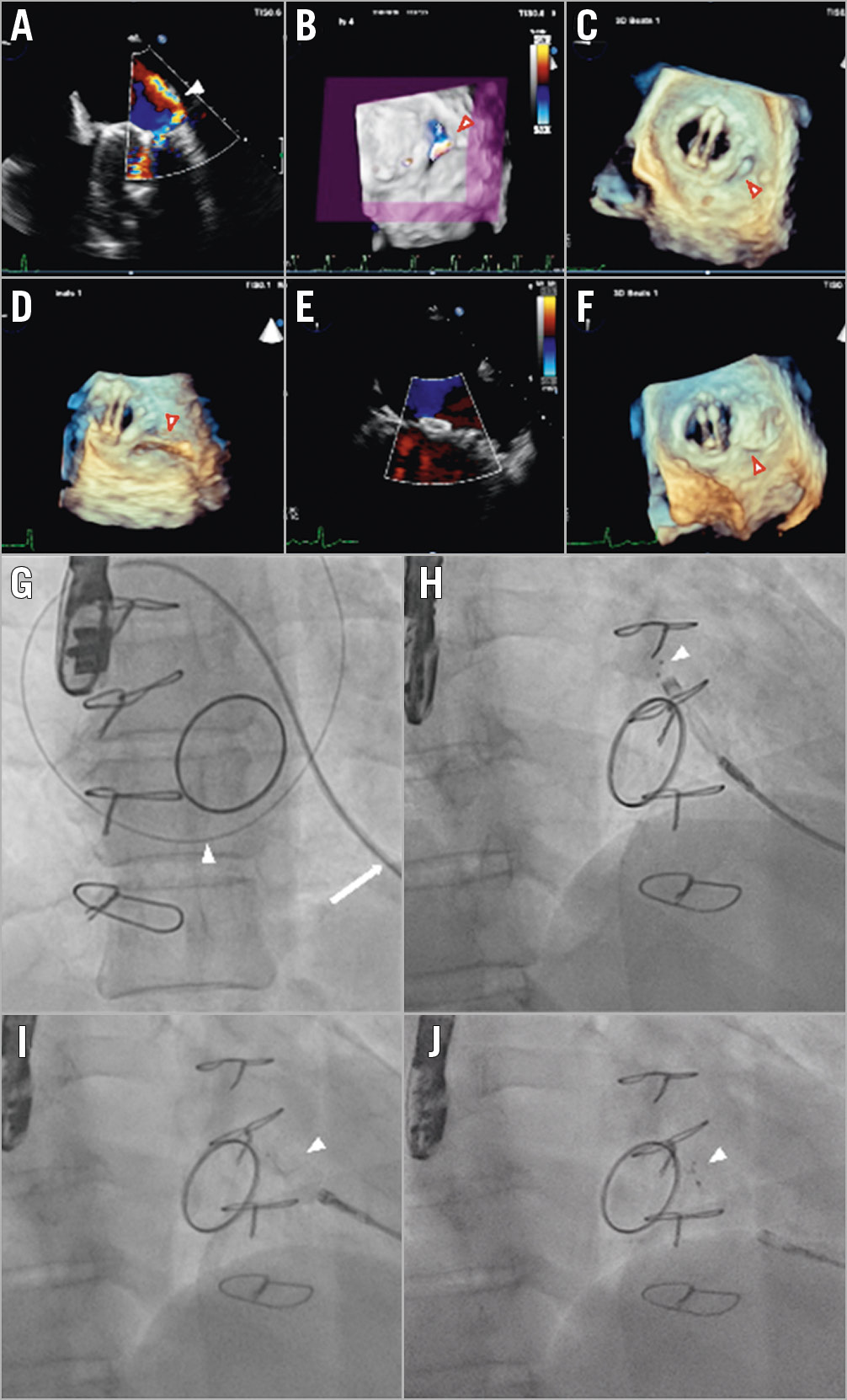
Figure 1. Echocardiographic (A-F) and fluoroscopic imaging (G-J) of transcatheter closure of a posterolateral, crescent-shaped mitral PVL with severe regurgitation in a patient with a bileaflet mechanical valve prosthesis. A) 2D TEE colour Doppler showing the regurgitant leak (arrowhead). B) 3D TEE colour Doppler image cropped at the level of the vena contracta clearly identified the single mitral paraprosthetic leak at 8 o’clock (arrowhead). C) 3D TEE showing the posterolateral defect (arrowhead). D) The wire crossing the PVL hole (arrowhead) seen on 3D TEE images. E) Post-procedure 2D TEE colour Doppler demonstrating no residual regurgitant leak. F) Final position of a 12×5 mm rectangular twist PLD (arrowhead) on 3D TEE. G) Fluoroscopic imaging showing a 5 Fr multipurpose catheter (arrow) and an exchange wire (arrowhead) crossing the mitral leak. H) Opening of the distal disc (arrowhead) of the occluder. I) Opening of the waist and the proximal disc (arrowhead) of the occluder. J) Full deployment of the 12×5 mm rectangular twist PLD (arrowhead) after delivery cable detachment.
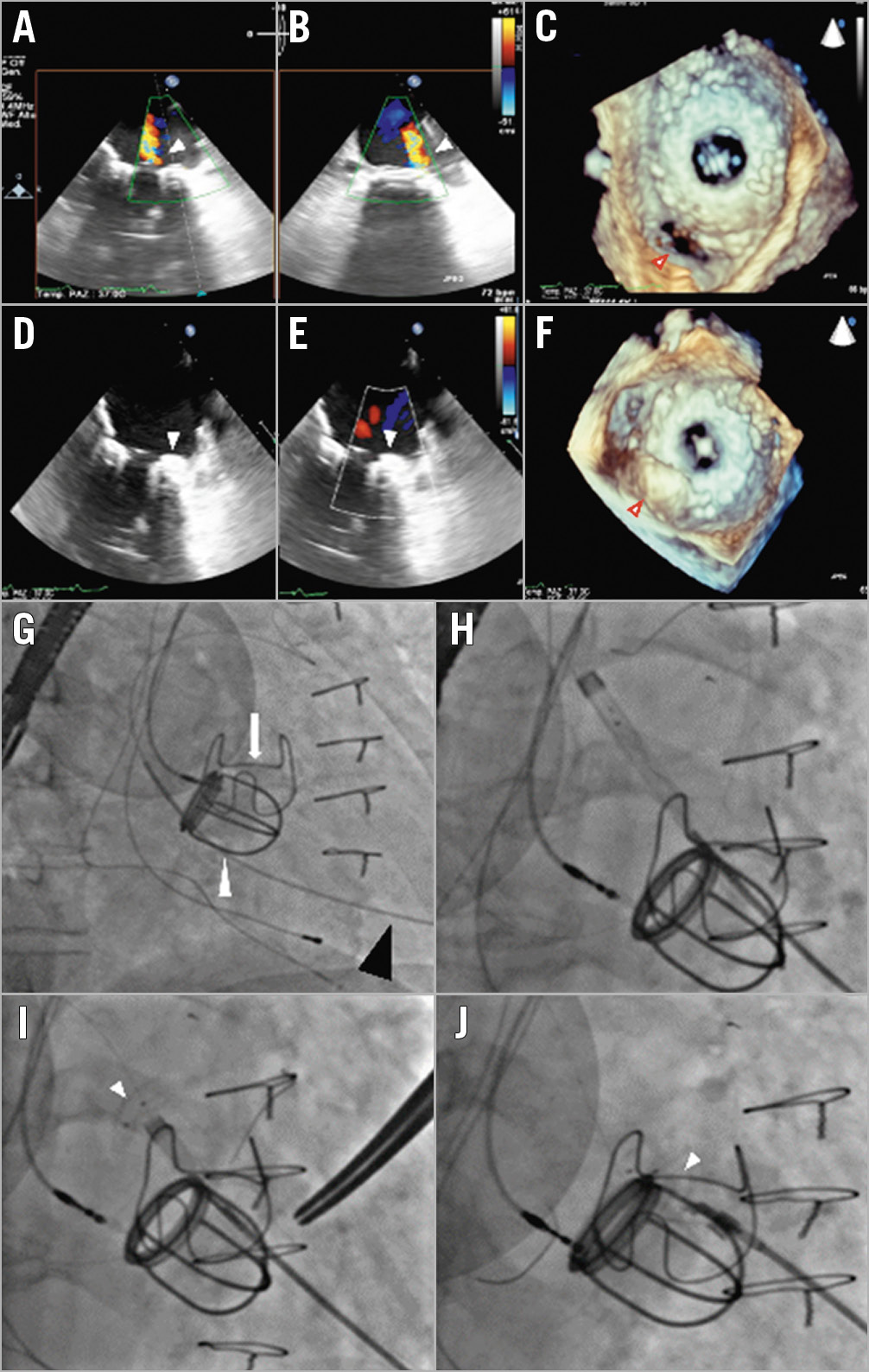
Figure 2. 2D colour Doppler and 3D TEE images before (A-C) and after (D-F) percutaneous closure of a crescent-shaped, posterolateral (7 o’clock) paravalvular mitral leak in a patient with a mechanical Starr-Edwards caged-ball prosthetic valve (arrowhead) and a mechanical aortic valve (arrow) + dual-chamber pacemaker. Fluoroscopic images (G-J) show the implantation steps of a 10×4 mm rectangular waist PLD.
Patients with a moderate-to-severe PVL causing heart failure and/or haemolysis with the need for recurrent blood transfusions who were deemed high risk for surgery by the Heart Team were considered for closure. Patients were treated according to the device instructions for use (IFU) and standard clinical practice. Procedures were performed under general anaesthesia (GA) or conscious sedation due to the need for intraprocedural TEE guidance. In a subset of patients, transapical catheter-based mitral PVL closure procedures were performed with a fusion of real-time 3D TEE and cardiac fluoroscopy imaging6.
THE OCCLUTECH PLD
The Occlutech PLD is a self-expanding, flexible, double-disc device made from nitinol-braided wires that has been specifically engineered combining and improving several features of previous off-label devices. Two different disc geometries are available, square and rectangular, connected by a waist of different sizes and shapes to improve stability and minimise the erosion risk of the surrounding tissue7,8.
STATISTICAL ANALYSIS
All statistical analyses were performed by means of commonly applied descriptive statistics. The study does not allow any confirmatory analyses. The analysis comprises the safety data of 136 patients. Baseline and six-month follow-up analyses consider data from 106 patients. Categorical variables are presented as numbers and percentages. Continuous variables are expressed as mean±standard deviation (SD) or median and interquartile range. Differences compared to baseline were assessed using a one-sample t-test for normally distributed variables. The Wilcoxon signed-rank test was used for non-normally distributed variables. Categorical variables were compared by the Wilcoxon signed-rank test. Two-sided p-values <0.05 were considered statistically significant in all analyses. All calculations were carried out with SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
A total of 179 PLDs were implanted in 136 consecutive patients in 21 centres in nine countries (December 2014–February 2018). Safety data were collected from 136 patients. Baseline and six-month follow-up data from 106 patients (69 mitral and 37 aortic) are considered in the following analyses, if not mentioned otherwise. The average patient follow-up time was 153.8±80 days.
BASELINE CHARACTERISTICS
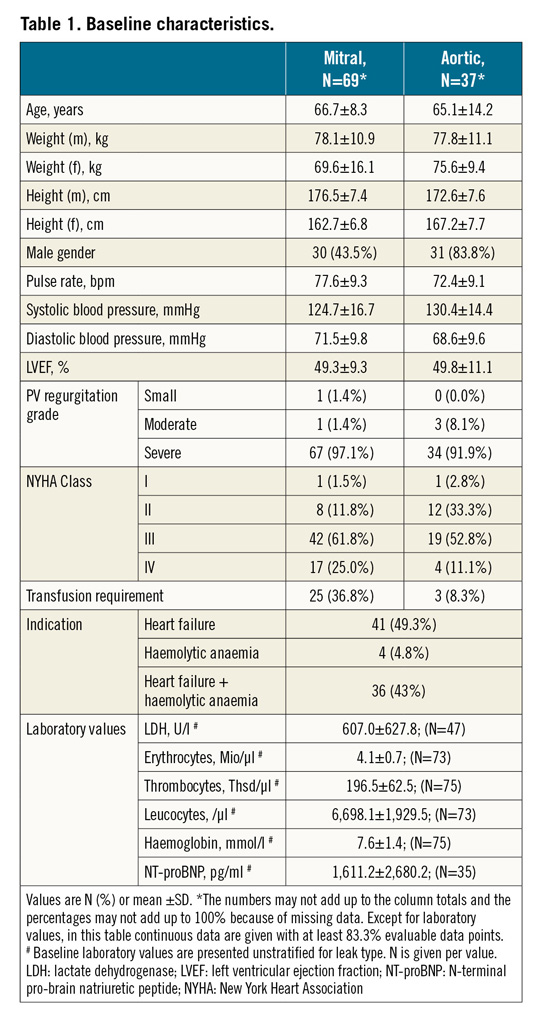
Demographic and clinical data for patients with ML and AL are summarised in Table 1. Mean patient age was 66.2 years (min: 26, max: 84), 58.1% were male and 78.9% presented in NYHA Class III/IV. Main indications were heart failure (49.3%), haemolytic anaemia (4.8%), or both (43%). Twenty-five (36.8%) of the ML and three (8.3%) of the AL patients were dependent on blood transfusions.
PROCEDURAL DETAILS
Table 2 shows procedural details. ML were approached under GA (62.5%) and 3D TEE guidance (63.1%) via surgical transapical (“hybrid approach”) (66.7%), antegrade transseptal (30.0%) or retrograde transaortic from the left ventricle (3.3%). In 20.6% of AL patients, PLD were implanted under GA and leaks were always accessed via a retrograde transaortic approach (100%). In 36.2% and 21.6% of ML and AL patients, respectively, multiple leaks were treated. Most ML and AL were closed with one PLD per leak (79.4% and 75.6%, respectively). In several patients, the number of PLD used was less than the number of leaks (10.3% in ML patients and 18.9% in AL patients). In most cases, rectangular waist shape PLD were used followed by the rectangular twist shape (Figure 3). Median procedural time for ML closure was 122.5 (110-135) minutes in transapical cases and 62.5 (48-125) minutes in transseptal cases. Median fluoroscopy time was not significantly different between the two access routes (20.5 vs 25 minutes). Median procedural and fluoroscopy times for AL closure were 90 (70-110) and 15 (11-24) minutes, respectively.
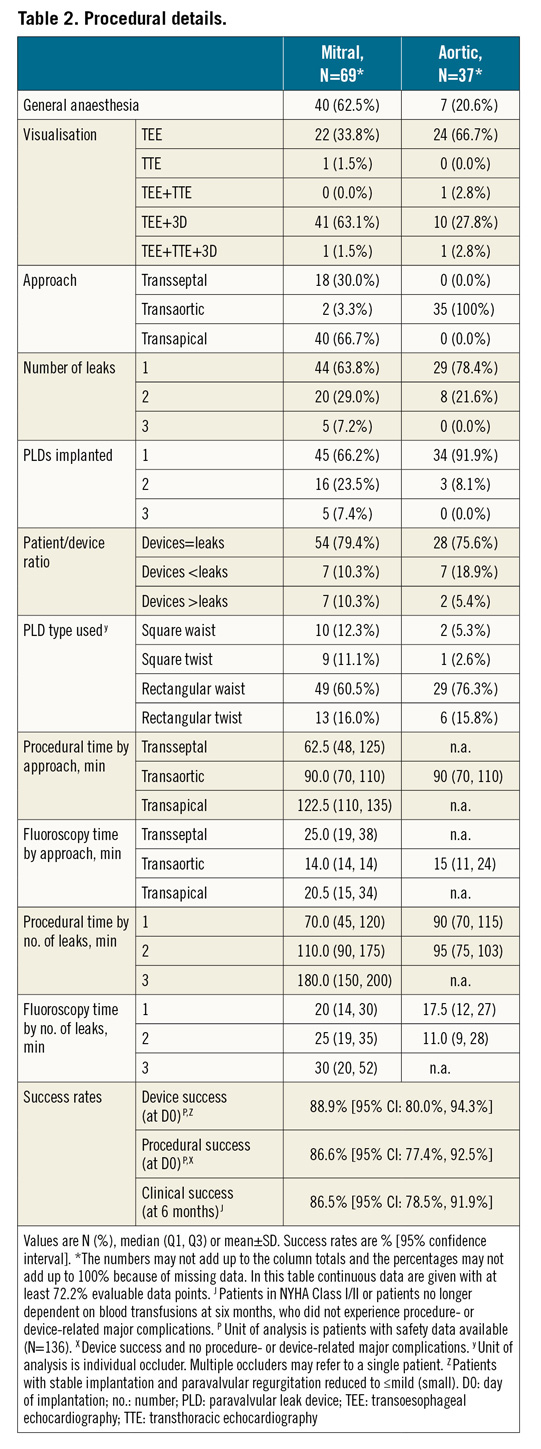
Figure 3. The Occlutech PLD types used for paravalvular leak closure with frequencies. Occlutech PLDs exist in two shapes (rectangular and square) and with two different types of connection between the proximal and distal disc (waist and twist). Frequencies are given. Unit of analysis is individual occluder.
LEAK CHARACTERISTICS
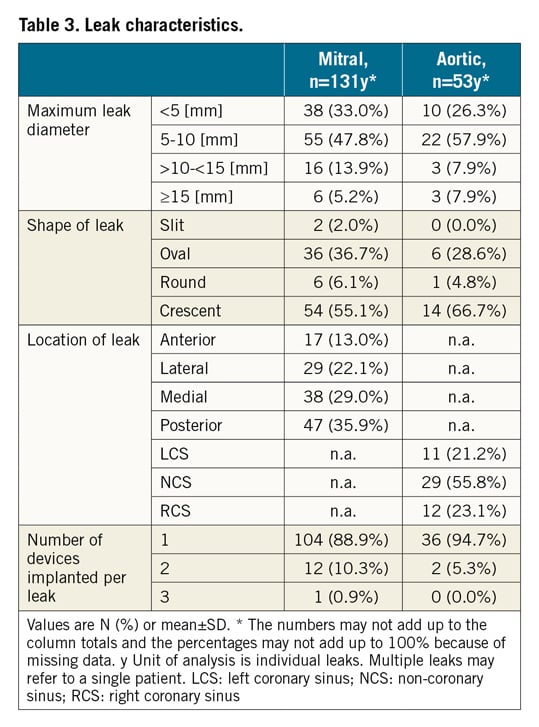
Table 3 presents details of the anatomical characteristics and number of PLD used in 131 ML and 53 AL patients. More than 80% of PVL had a maximum diameter of <10 mm and either a crescent or oval shape. ML were located posteriorly in 35.9% of the cases and in a medial position in 29%. Most AL were located in the non-coronary sinus area (55.8%). Intraprocedural TEE showed severe PV regurgitation in 97.1%, moderate in 81.4% and small in 1.4% of ML patients, and severe in 91.9% and moderate in 8% of AL patients.
OUTCOMES
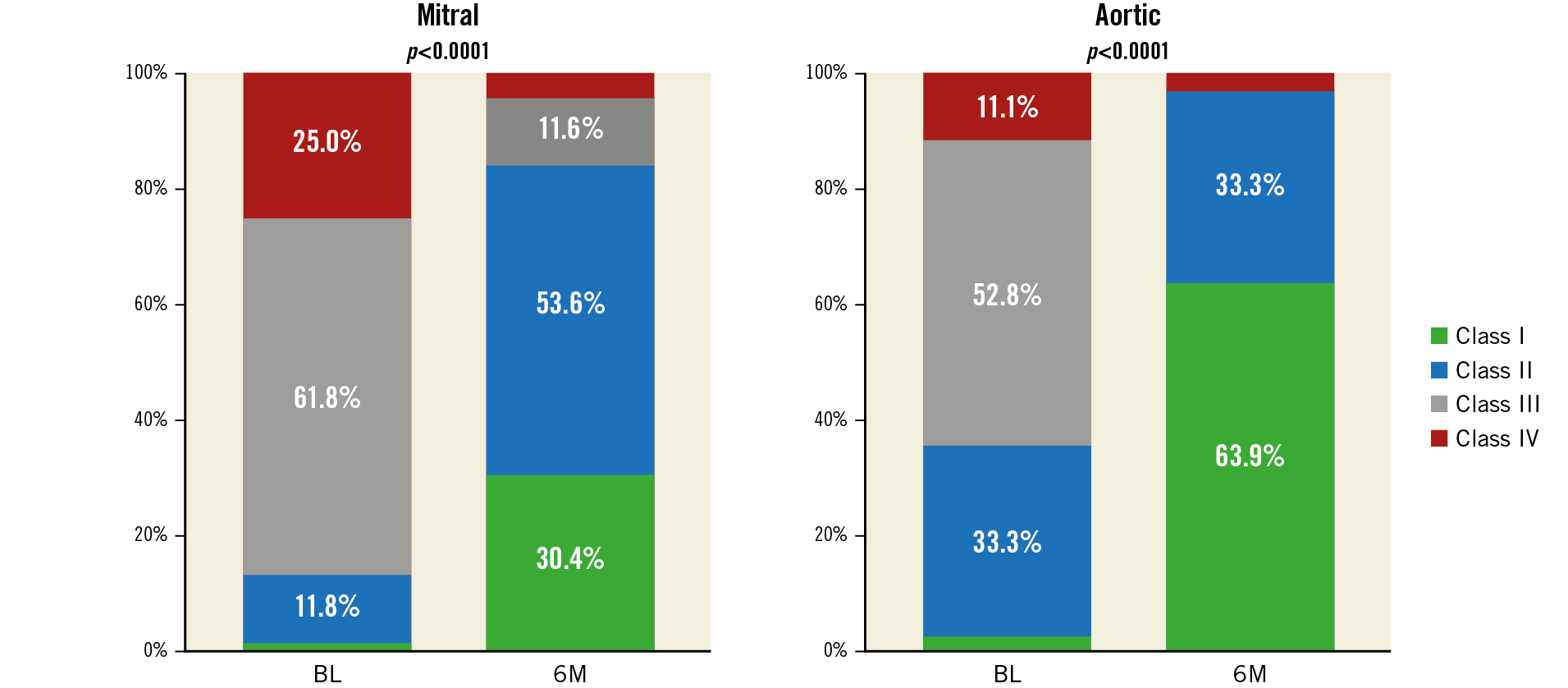
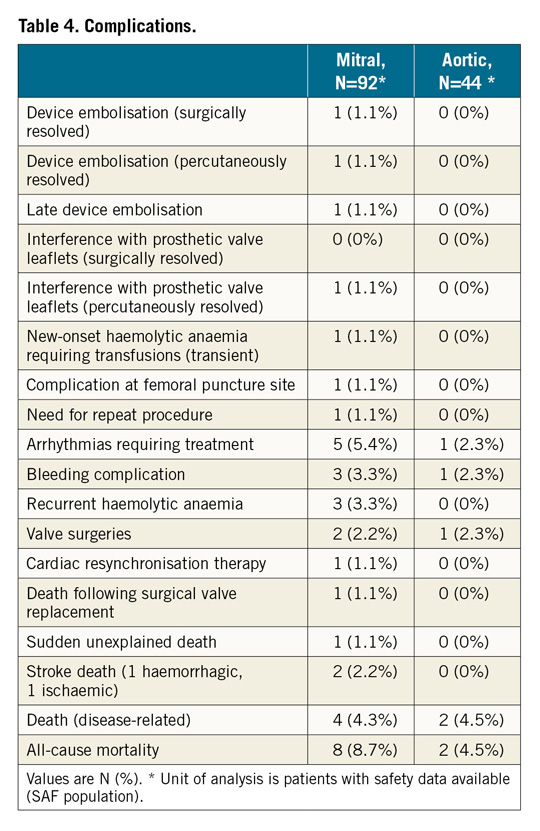
At follow-up, paravalvular regurgitation was severe in 4.5%, moderate in 7.6%, small in 56.1% and absent in 31.8% in ML patients. In AL patients, it was severe in 2.7%, moderate in 10.8%, small in 81.1% and absent in 5.4% (Figure 4). Overall, PVL improved from moderate/severe to no more than mild/small in 87.7% of ML patients and 86.5% of AL patients. One (0.7%) patient underwent repeat closure four months after the index procedure because of significant residual leak. The device success rate, defined as stable implantation and PV regurgitation reduction to Figure 4. Paravalvular regurgitation in the mitral and aortic patient populations before and six months following implantation. Wilcoxon signed-rank test has been applied. Fractions without percent value represent mitral leaks at BL (small [1.4%] and moderate [1.4%]) and at 6M (moderate [7.6%] and severe [4.5%]), and for aortic leaks at BL (no [0%] and moderate [8.1%]) and at 6M (no [5.4%] and severe [2.7%]). BL: baseline visit; 6M: six-month follow-up visit NYHA class improved in most of the patients over a mean follow-up of 153±80 days. The proportion of patients in NYHA Class III/IV decreased from 86.8% at baseline to 11.4% at follow-up (Figure 5). The proportion of patients in need of haemolysis-related blood transfusion decreased from 36.8% to 5.9% and 8.3% to 0% in ML and AL patients, respectively. The laboratory values of a subset of patients are summarised in Supplementary Figure 1. The clinical success rate, defined as patients in NYHA Class I/II or no longer dependent on blood transfusions at six-month follow-up, was 86.5%. All-cause mortality was 7.4%. No death was associated with the device. Figure 5. NYHA functional classification in the mitral and aortic patient populations before and six months following implantation. Wilcoxon signed-rank test has been applied. Fractions without percent value represent mitral leaks at BL (Class I [1.5%]) and at 6M (Class IV [4.3%]), and for aortic leaks at BL (Class I [2.8%]) and at 6M (Class IV [2.8%]). BL: baseline visit; NYHA: New York Heart Association; 6M: six-month follow-up visit One (0.7%) patient with a small residual leak had recurrence of haemolytic anaemia requiring transient blood tranfusions. In another case, intraprocedural TEE showed that PLD deployment blocked the movement of the prosthetic valve leaflets. The device was recaptured percutaneously without any complication and successfully replaced with a smaller PDL. Two intraprocedural embolisations occurred during delivery sheath placement for the deployment of an additional PLD. One embolisation was managed surgically and one with percutaneous retrieval. One late embolisation was detected at follow-up. Complications at the femoral puncture site occurred in 0.7%, bleeding complications in 2.9% and arrhythmias requiring treatment in 4.4% of the cases. In 66.7% of the patients, arrhythmias occurred during the procedure or hospital stay (Table 4). Discussion The performance endpoint of this study was effective PVL closure, defined as a stable implantation and PVL reduction to no more than mild. The study met this endpoint in 88.9% of the patients. These results compare favourably with those of previous studies, which showed technical success rates ranging between 62%9 and 87%10. In 87.3% of our study patients, PVL was mild or no longer detectable at six-month echocardiography follow-up. This result is of utmost importance as several studies have shown a direct correlation between the grade of residual regurgitation and the rate of repeat intervention and survival3,11,12,13. In line with the technical success observed, PVL closure was associated with significant clinical improvement and reduction of the need for blood transfusion. As with every interventional technique, transcatheter PVL closure is not free from potential complications14,15. Device malpositioning or embolisation has been reported in 1% to 5% of large series12,16. The main causes are frail tissue around the valve, multiple device deployment and complicated access to PVL combined with imaging limitations. Generally, in such cases, snaring and recapture of the device into the delivery sheath can be performed. Only one (0.7%) embolisation requiring surgery was reported in our registry. Interference on prosthetic valve leaflet function is a feared complication of percutaneous PVL closure. It is not rare: clinical studies report rates ranging from 3.6%3 to 5%16,17. In our registry, this occurred in one (0.7%) patient only. Importantly, interference occurred during the procedure and before device release. The very low rate of valve interference may be attributed to the unique design of the PLD, whose concavities of the four edges produce only minimal overlapping with the surgical valve. Undoubtedly, careful image-based assessment of PVL anatomy is of utmost importance for choosing the right size of device and for ensuring the proper apposition of the device disc to the surrounding tissue with full sealing18. Of note, oversized devices might also have the opposite effect and increase the regurgitant defect. Regurgitation through a residual leak or the PLD has an important impact on clinical outcome. Indeed, it can be associated with persistent or new haemolysis. We observed new haemolytic anaemia in one (0.7%) patient and recurrence of haemolytic anaemia in 2.2% of the patients. In two additional patients, who underwent successful PVL closure for haemolytic anaemia, transfusion dependency was reduced but not completely avoided. With a moderate or severe residual leak observed in 12.1% of the ML patients and 13.5% of the AL patients, the Occlutech PLD compares favourably with other devices for which moderate or severe residual leaks were reported in 11% to 24% of the cases3,11. A repeat procedure was needed in one (0.7%) patient only, a rate lower than that (6%) reported by Calvert et al19. Most of our patients showed clinical benefits, as indicated by a significant improvement of NYHA class and a significant reduction of haemolytic anaemia and haemolysis-related blood transfusions. It must be emphasised that the percutaneous PVL closure procedure is performed mostly in high-risk patients for whom repeat surgery is not suitable. Accordingly, most patients with PVL have multiple comorbidities, underlining the need for a less invasive procedure. Indeed, the all-cause mortality rate of 7.4% observed in our registry is comparable to that observed in the literature3,19 and may be explained by the high-risk characteristics of the patients. It should be noted that a significant improvement in procedural outcomes has been reported with increasing operator experience12, underlining the importance of the learning curve associated with this complex procedure, which requires commitment and a wide variety of interventional skills. In summary, the choice of an appropriate occluder device along with thorough preprocedural planning using advanced imaging modalities (specifically fusion imaging) and alternative access approaches (transapical “hybrid technique”) are critical for achieving a high intraprocedural success rate and for reducing major adverse events of this transcatheter procedure. Study limitations This is a retrospective registry. Therefore, there is a theoretical bias associated with such an investigation. There was no evaluation of TEE by a central core laboratory, nor was an audit of the records performed. Having many different centres and investigators with different skills and techniques participating in the study increases the complexity of comparison of the implantation methods used. Finally, there was no control group comparing this treatment to surgery. Conclusions Transcatheter PVL closure with a specifically designed PLD demonstrated effectiveness with a relatively low rate of major complications. Procedural success for ML and AL closure was high, with a low rate of residual or recurrent leaks, and was associated with significant improvement in NYHA class and reduction of haemolytic anaemia and transfusion dependency. However, further data are needed to assess the clinical outcome of patients treated with this device at longer-term follow-up. Impact on daily practice Acknowledgements The authors would like to acknowledge all participating investigators who are listed in Supplementary Appendix 1 for making it possible to perform this international study. The authors express their appreciation to Simon Kordowich and Thomas Schöndorf for providing statistical support. Conflict of interest statement E.M. Onorato is a consultant for Occlutech. The other authors have no conflicts of interest to declare. To read the full content of this article, please download the PDF. Moving image 1. 2D TEE colour Doppler showing a significant regurgitant jet through a mitral paravalvular leak. Moving image 2. 3D TEE colour Doppler showing an anterolateral (9-11 o’clock) mitral paravalvular leak. Moving image 3. Real-time 3D TEE showing the crescent shape of the mitral leak at 9-11 o’clock. Moving image 4. Fluoroscopic (A) and real-time 3D TEE showing the catheter crossing the leak (arrows) from a transapical approach. Moving image 5. Procedural fluoroscopic steps of the PVL closure procedure using a 12×5 mm rectangular waist paravalvular leak device (PLD). A) Distal disc opening; B) waist and proximal disc opening; C) stability test (“pull & push”); D) 12×5 mm PLD in situ. Moving image 6. 3D TEE colour Doppler in two different views showing final position of the occluder device.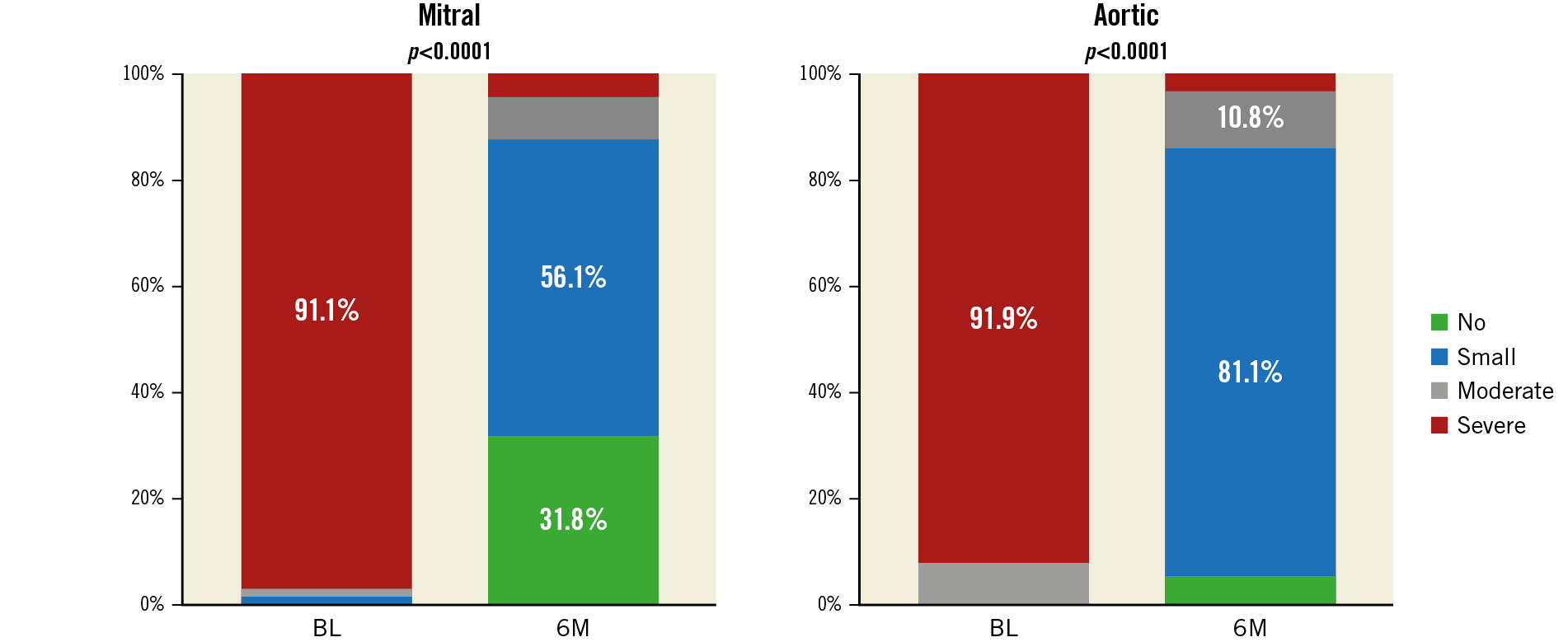
Paravalvular leak is an important complication of valve replacement surgery and is associated with significant morbidity and mortality. For high-risk symptomatic PVL patients, catheter closure is a viable therapeutic alternative strategy to surgical PVL repair and may represent a first-line treatment. Transcatheter PVL closure with the specifically designed Occlutech PLD occluder was demonstrated to be an effective procedure with a relatively low rate of serious complications.Supplementary data

