
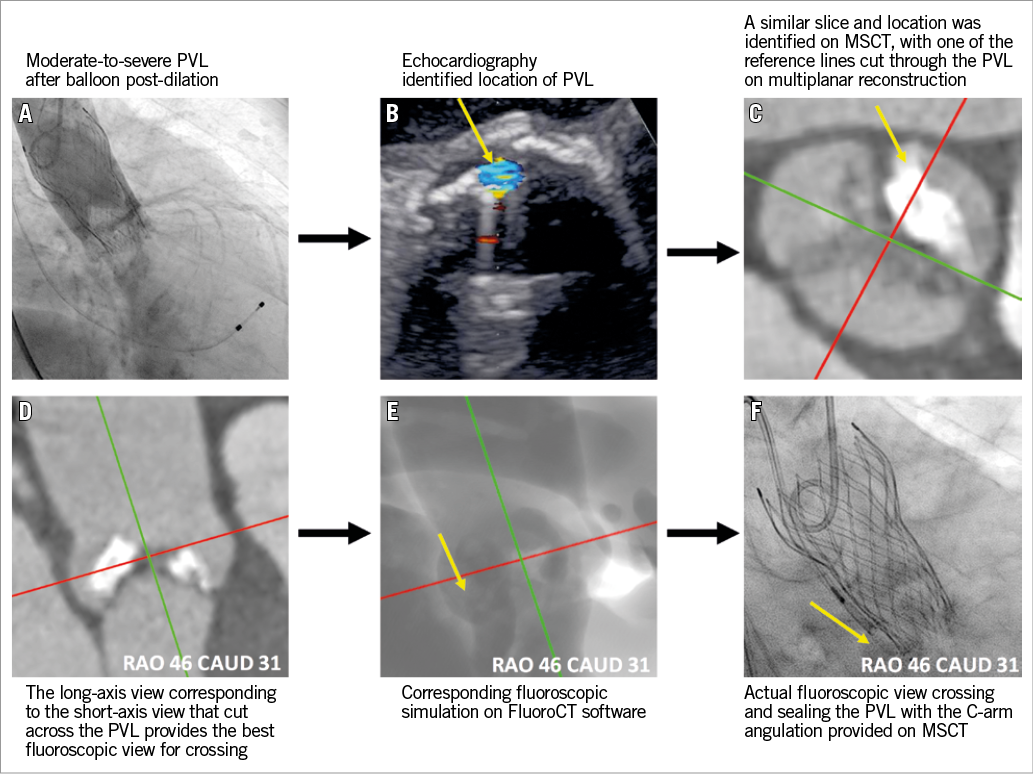
A 91-year-old female with symptomatic severe aortic stenosis (aortic valve area 0.4 cm2, transvalvular mean gradient 77 mmHg) was referred for transcatheter aortic valve replacement (TAVR). A 26 mm CoreValve® Evolut™ R bioprosthesis (Medtronic, Minneapolis, MN, USA) was planned with a perimeter oversizing of 14.6%. A large calcium nodule was noted at the non-coronary cusp on multislice computed tomography (MSCT). Moderate-to-severe paravalvular leak (PVL) was noted on post-deployment aortography. Post-dilation with a 22 mm balloon failed to improve the amount of PVL (Panel A). Considering the significant risk of suboptimal stent apposition and conformability to the surrounding anatomy, even after aggressive post-dilation, and the concern of aortic root perforation/rupture, we chose percutaneous PVL closure to seal the leak.
According to the intraprocedural transoesophageal echocardiogram (TEE), the regurgitant jet was located at the commissure between the left and non-coronary cusps (Panel B). According to the location of the regurgitant jet on the TEE images, we selected an optimal fluoroscopic viewing angle (RAO 46, CAUD 31) for crossing the paravalvular leak after appropriate multiplanar reconstruction of the pre-TAVR MSCT (Panel C-Panel F), using FluoroCT software (Circle Cardiovascular Imaging Inc., Calgary, Canada). The leak was passed with a 0.035-inch J wire, through which an 8 Fr multipurpose guiding catheter crossed the defect. The selected 8 mm AMPLATZER™ Vascular Plug II (St. Jude Medical, St. Paul, MN, USA) was loaded into the delivery system, advanced through the multipurpose guiding catheter and deployed. Position and closure of the leak were confirmed by TEE and angiography. Of note, only 12 minutes were required to analyse the TEE and MSCT images, and cross/plug the PVL.
Percutaneous PVL closure after TAVR is a technically demanding procedure with unpredictable results. Three-dimensional TEE imaging has been shown to benefit such procedures in preprocedural defect sizing, device selection and deployment guidance1. Crossing the PVL defect, however, can still be time-consuming or require exchanges of catheters and guidewires2. The source of these challenges can be difficult anatomies, suboptimal imaging techniques and C-arm angulations. The difficulty in defining the crossing angulation can be easily solved with MSCT while using TEE to identify the location of the defect. Integrated multimodality imaging using echocardiography, MSCT and fluoroscopy was critical to the success of this procedure.
Conflict of interest statement
N. Piazza is a consultant/proctor for HighLife, Medtronic and MicroPort, and is a consultant for Cephea. The other authors have no conflicts of interest to declare.

