
CASE SUMMARY
BACKGROUND: A few weeks after complicated redo bioprosthetic aortic valve replacement for infective endocarditis of a bioprosthesis, a 74-year-old male patient developed severe and symptomatic aortic valve regurgitation due to a large paravalvular leak (PVL) and underwent successful interventional closure of the PVL with sequential implantation of four AMPLATZER Vascular Plug III devices. Despite the initial success of the procedure, with a significant decrease in AR (grade II) and clinical stabilisation of the patient, he was re-admitted to hospital six months later due to advanced signs of heart failure. 3D-TOE again showed severe paravalvular AR next to the implanted plugs.
INVESTIGATION: Transthoracic echocardiography, 3D-TOE, laboratory tests, cardiac catheterisation including aortography.
DIAGNOSIS: New severe PVL after interventional PVL closure after complicated bioprosthetic aortic valve replacement.
MANAGEMENT: Interventional PVL closure.
KEYWORDS: catheter-based closure, interventional closure, paravalvular leak, plug
PRESENTATION OF THE CASE
In July 2014, a 74-year-old male patient in good general condition underwent a surgical aortic valve replacement (SAVR) with xenograft, mitral and tricuspid valve reconstruction due to severe symptomatic aortic valve regurgitation due to prolapse of the right and left coronary cusp, moderate to severe secondary mitral valve regurgitation and severe tricuspid valve regurgitation. Severely reduced ejection fraction was present on echocardiography before the procedure. After an uneventful early postoperative period with regular discharge, the patient was re-admitted to hospital three weeks after the procedure for symptoms of stroke. Cerebral MRI showed multiple cerebral ischaemic lesions in different areas of the brain, suggesting a cardioembolic origin. Acute endocarditis of the reconstructed mitral valve and the prosthetic aortic valve was confirmed by transoesophageal echocardiography (TOE) and detection of coagulase-negative staphylococcus in all blood cultures. Four weeks after the first surgical valve replacement, the patient underwent re-operation including implantation of a 27 mm xenograft in the aortic valve position and a Hancock® II prosthesis (xenograft, 33 mm) (Medtronic, Minneapolis, MN, USA) in the mitral valve position. During the operation, a paravalvular leak next to the aortic bioprosthesis was observed by the surgeon but was left untreated due to a complicated situs. After a good initial recovery, the patient developed progressive signs of congestive heart failure a few weeks after re-operation due to severe paravalvular aortic valve regurgitation. 3D-TOE showed a paravalvular defect at 7 o’clock (according to the location diagram of Ruiz et al1) with an orifice diameter of approximately 13×8 mm (Figure 1, Figure 2). No signs of re-endocarditis could be detected on TOE or in blood cultures. Because of a substantial mortality risk during re-re-operation (log EuroSCORE I=39%) and surgical closure of the paravalvular leak, the local Heart Team favoured an interventional approach for paravalvular leak closure. A hydrophilic guidewire was used to cross the defect via a retrograde femoral arterial access, and a 5 Fr catheter was inserted into the left ventricle (LV). Afterwards, the hydrophilic guidewire was exchanged for an extra-support wire and the delivery sheath was inserted into the left ventricle. Through the delivery sheath, the distal part of the plug device was released into the LV, pulled back into the paravalvular defect, and then the proximal part of the occluder system was released at the aortic side (Figure 3). Due to continuing severe aortic insufficiency, three further AMPLATZER™ Vascular Plug III (AVP III) devices (St. Jude Medical, St. Paul, MN, USA) (Figure 4) were implanted simultaneously and released to close the large paravalvular defect (14×5 mm, 10×5 mm, 8×4 mm). For persisting relevant paravalvular regurgitation, a fourth AVP III device was successfully implanted sequentially (12×5 mm) (Figure 5). As a result, a reduction of the paravalvular aortic valve regurgitation from grade IV to grade II and an elevation of the diastolic aortic pressure from 30 to 55 mmHg was immediately observed. After the procedure, the patient showed marked clinical relief of dyspnoea and was able to be discharged from hospital a few days after.
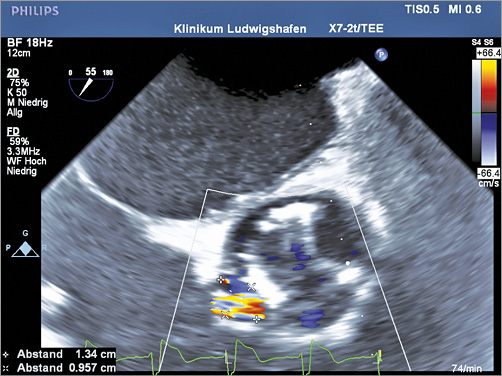
Figure 1. 2D-TOE showing the location and diameter of the paravalvular leak in relation to the implanted bioprosthetic aortic valve.
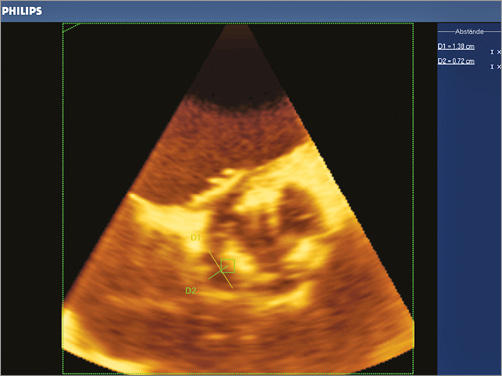
Figure 2. 3D-TOE showing the location and diameter of the paravalvular leak in relation to the implanted bioprosthetic aortic valve.
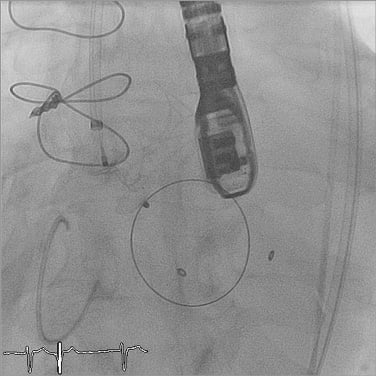
Figure 3. Implantation of the first AVP III into the paravalvular dehiscence. At this stage the plug device is still connected to the introducer system and ready for final release.

Figure 4. AMPLATZER Vascular Plug III (St. Jude Medical).
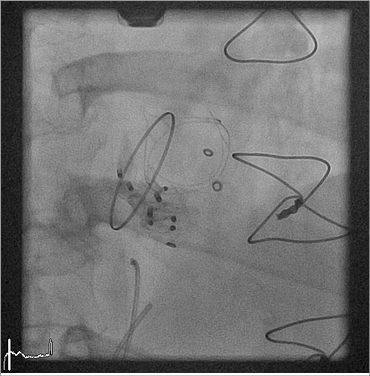
Figure 5. Final result after implantation of four AVP III devices into the paravalvular leak.
Six months later, our patient was re-admitted to hospital for congestive heart failure. 3D-TOE showed a new paravalvular defect next to the previously implanted vascular plugs (at 8-11 o’clock) with severe aortic regurgitation (Figure 6). The amount of previously implanted artificial material with multiple artefacts on 3D-TOE made an exact sizing of the defect by TOE almost impossible. On the one hand our patient had a very high surgical risk, while on the other a second interventional approach carried a substantial risk of technical failure or complications, such as device embolisation, without knowing the size of the paravalvular defect. This treatment dilemma was discussed in our local Heart Team conference.
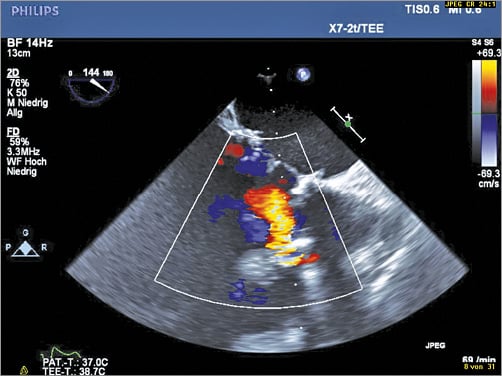
Figure 6. New and clinically relevant eccentric paravalvular aortic regurgitation in colour duplex (2D-TOE).
How would I treat?
THE INVITED EXPERT’S OPINION

The case represents a rare but not uncommon condition. This is a 74-year-old man with two sternotomies and now resultant 27 mm xenograft in the aortic position and bioprosthetic mitral valve regurgitation (MVR) with prior TV reconstruction. A paravalvular leak was left behind at the time of second surgery due to a complicated situs; this was followed by severe paravalvular leak (PVL) in the subacute postoperative course. As the authors mentioned, this was probably not due to infectious aetiology (present at the time of surgery and negative cultures). For a leak of 13×8 mm, four AVP III devices were placed in the aortic position. Six months later, a new paravalvular leak adjacent to the devices was seen, but now a new leak is seen, presumably in the area of the right or non-coronary cusp next to the devices, based on Figure 6.
One interesting point is the use of four devices for a single leak of 13×8 mm. This is quite a significant number and, although there is a degree of compression associated with the AVP III devices, the fluoroscopy image (Figure 5) shows that the devices are more compressed, with at least two of the devices “on top of each other”. Despite four devices, the regurgitation decreased only to grade II, which raises suspicion that the leak extended as a result of device manipulation and expansion in the leak orifice. This may also be due to poor tissue integrity, which is often the case during infectious aetiology or in patients on chronic steroid use.
Paravalvular leak closure late “failure” at six months can happen for a variety of reasons. This may be due to infection, late embolisation/migration, or leak extension. If infection is the cause here (blood cultures should be obtained and vegetation should be excluded), then surgery is the logical choice and should be reconsidered. A percutaneous therapy is likely to become infected as well. Late embolisation/migration can be evaluated by fluoroscopy (comparing to Figure 5) and also by 3D-TOE. If the device has embolised, it can often be retrieved by percutaneous methods. Most often it will be present at the aortic bifurcation or be in the pelvic or femoral vasculature; this may be with or without symptoms of claudication. Bioptome or snare removal technique may then be used. These patients may be candidates for re-attempt at leak closure in that location with a larger device or a similar device placed slightly differently. If there is extension of the leak (due to poor tissue integrity and/or torn suture), one could consider another closure but the same extension issue may also happen with a second closure attempt.
For this patient, I would consider surgery as a first option, medical management as a second option, and interventional closure as a third option. The reason is that one percutaneous attempt has already been made without significant long-standing results. It is time to go back to the drawing board and see if surgery is fully off the table in this 74-year-old man. If surgery is completely ruled out, then I would consider a course of medical management. Our position as interventionalists is often to look for a possible interventional solution; this is a patient where a prior intervention has not given long-lasting benefit. Medical management with diuretics, permissive tachycardia, and very close phone/outpatient follow-up may provide significant symptomatic relief. If the patient has significant symptoms and hospitalisations despite this, a further percutaneous step may be necessary.
Before a second percutaneous attempt, measurement of the deficit is the main question. This can be performed by computerised tomography (CT) measurements2-4 or by intraprocedural angiography5. Although there will probably be artefact from the other devices on CT scan, this can be mitigated by optimising pre-scan drug therapy, optimum window and centre adjustments, and careful selection of valve planes4. Careful evaluation of three-dimensional vena contracta on TOE can provide clues as to the location of the defect and this can be correlated with angiography and computerised tomography. In addition, 3D printing may be very useful here for planning6, but needs a useful CT data set to be successful. The advantage of 3D printing is that device implantation may be attempted in vitro; however, this does not mimic changes that may happen during the cardiac cycle. Utilising all of these methods, accurate sizing of the defect may be possible.
Despite accurate measurement, other challenges and opportunities exist. The accuracy of the measurement also does not reflect the possible serpiginous nature of the defect, as well as the integrity of the tissue in this location. Careful placement of an AVP IV (St. Jude Medical) or a small plug could be considered, but the long-term result of this method would be tough to predict, given the prior failure at the six-month mark. This would be a first percutaneous option if possible, and could be performed using the same transfemoral approach as per the prior cases. It would be important to note whether the defect extends further during sheath/device manoeuvring and manipulation, in which case the operators should consider stopping the procedure at that point.
Another option would be to place a TAVI valve inside the native bioprosthesis. The principle behind this would be that radial force from the TAVI valve on the bioprosthetic leaflets may help to compress the existing plugs further, helping to seal the leak (the native bioprosthesis is of course not likely to expand further). A balloon occlusion test with concomitant aortic angiography may be helpful (to check for decrease in aortic regurgitation as well as patency of the coronary arteries). If this shows benefit, data from this manoeuvre, as well as a TAVI-type CT scan, may be helpful to see if this patient is a candidate for a balloon-expandable, self-expanding, or mechanically expanding valve. There may be advantages to the intrinsic radial force of the valve, sealing cuff, and recapturability. Radial force of the TAVI valve may push the leaflets out further and mimic the balloon occlusion test. A sealing cuff could mitigate the leak further (especially if placed low enough; this would have to be balanced with the interaction with existing plugs and possible overhang of the surgical leaflets). Repositionability/recapturability would allow fine-tuning and bail-out if needed. Other issues, such as interaction with the mitral bioprosthesis and the patient’s full understanding of the risks and benefits should also be examined further before moving forward. This is less likely to be successful but, in a case like this, I would evaluate a CT for this option as well.
This is, as mentioned earlier, a rare but not uncommon case. As the authors mention, patients need close follow-up after paravalvular leak closure. Subacute and chronic failure can happen for a variety of reasons, despite acute procedural success. Surgical correction and medical management should always be (re)considered in patients after failure of a percutaneous attempt. If further percutaneous attempts are needed due to lack of a surgical option and failure of medical management, close evaluation of leak size and shape can be made by a combination of echocardiography, computerised tomography, angiography, and 3D printing. Closure may be performed using a combination of leak closure and, potentially, the sealing cuff of a TAVI valve; however, this should be evaluated closely before further attempts are made.
Conflict of interest statement
S. Gafoor is a consultant or proctor for Medtronic, Abbott Vascular and Boston Scientific.
How would I treat?
THE INVITED EXPERT’S OPINION

The authors report on a complex case of paravalvular leak after surgical implantation of a 27 mm xenograft in the aortic valve position in a 74-year-old male patient. He had a complex previous history with multiple surgical procedures, bacterial endocarditis and substantial mortality risk (log EuroSCORE I=39%). Because of the development of a significant paravalvular leak three weeks after the last surgery, he was proposed for a transcatheter approach and underwent sequential implantation of four AVP III (14×5 mm+10×5 mm+8×4 mm+12×5 mm) devices. After the procedure, a significant reduction of paravalvular aortic leak occurred, NYHA Class reduced from IV to II, and aortic diastolic pressure increased from 30 to 55 mmHg.
Unfortunately, six months later he was readmitted for congestive heart failure because of a new paravalvular defect next to the previously implanted devices.
There are some relevant points that should be clarified before considering any option:
1) Do the devices look stable on fluoroscopy or do they look “moving”?
2) Are we sure that the infective process is completely cured? Are there signs of active endocarditis? What about C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), blood count? Could a scintigraphy with marked leucocytes be useful to rule out a still ongoing infection?
Presuming that the two previous points give positive answers, I think that the first option could be surgery. However, some interventional approaches are also possible.
Three arterial vascular accesses are needed. The first step is the protection of the brain from potential embolisations by using a filter commonly employed during aortic valve implantation (Sentinel™; Claret Medical, Inc., Santa Rosa, CA, USA).
The second step is to stabilise the “unstable” devices by using a gooseneck snare(s) as shown in one of our previous papers7.
The third step is to “interrogate” the residual leak by balloon while performing an aortography to rule out a significant residual leakage when the balloon is through the leak. First, I would use a 24 mm AMPLATZER™ ASD sizing balloon (St. Jude Medical). As a further step, I would use an angioplasty balloon (Cristal; Balt, Montmorency, France) 2-4 mm larger than the size found by the ASD sizing balloon.
In fact, an ASD sizing balloon does not produce compression, while, by using an angioplasty balloon, a somewhat “gentle” compression (2-3 atmospheres) can be applied, showing whether the residual leak is closed. In fact, simultaneous angiographies in the ascending aorta will show how the residaul leakage behaves. The balloon is deflated, the leak checked and device stability ascertained.
The fourth step is device choice and placement. According to the size of the residual defect, I would go for an AVP II (St. Jude Medical) or an AMPLATZER™ Muscular VSD Occluder device (St. Jude Medical). Probably my preference would be to go for a Muscular VSD device. In fact, here we already have some materials inside the leak; therefore, we wish to have a device exerting some pressure around it and, more importantly, a device with fabric inside to promote quick occlusion.
An alternative, probably “crazy” option, would be to implant a covered stent (Atrium Advanta™; Maquet Getinge Group, Rastatt, Germany, or a Covered CP Stent™; NuMED, Hopkinton, NY, USA) 5 to 10 mm longer then the prosthesis. Therefore, the preferred length would be 24-29 mm because the height of the implanted xenograft is 19 mm. The stent would be implanted at the diameter where the residual leakage disappears or becomes trivial. This is evaluated in the previous step by using balloon “sizing” and ascending aortographies. Then the balloon is deflated, leaving in place the covered stent that could be easily occluded by an AVP II.
At any stage, surgery should be available.
Conflict of interest statement
G. Butera is a proctor for St. Jude Medical/Abbott.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
Interventional closure of paravalvular leak is a relatively new and promising treatment option for affected patients, in whom morbidity and mortality risk at open re-surgery is frequently increased. To date, only sparse data on interventional closure of PVL are available, operators are still learning and the optimal technical strategy is still to be defined. Most available data focus on the in-hospital follow-up. Here, complication rates were acceptably low, while technical success rates were high. To date, all plug devices used for interventional closure of PVL have originally been designed for different indications such as vascular closure and closure of ventricular septal defects, and are consequently used off-label for leak closure. However, a limited variety of plug devices with different shapes and sizes is available and has been successfully used at interventional closure of PVL in the past8. Paravalvular defects usually show an irregular shape in 3D-TOE, which often makes it difficult to select plug devices to close the defect optimally. In particular, the exact sizing of large oval or crescentic paravalvular defects is often challenging. Due to its special elongated shape, the AVP III (also used in the case report) is particularly suitable for interventional closure of oval or crescentic paravalvular defects (Figure 4). Accurate sizing is of paramount importance for technical success to avoid device embolisation due to undersizing or device interference with the prosthetic valve due to oversizing. In large paravalvular defects (such as in our clinical case), it is often necessary to implant multiple plug devices to reduce the grade of paravalvular regurgitation successfully. The deployment of multiple plug devices can be performed either simultaneously by using two or more delivery systems or sequentially by implanting one plug after the other using the same delivery system8. In our case, four plug devices (three simultaneously and one sequentially) needed to be implanted to reduce the severe paravalvular regurgitation to a moderate grade in a first procedure. After the first implantation of four vascular plugs, our patient was discharged in a stable clinical condition and was encouraged to show up regularly for clinical follow-up visits at our site, which he did not do afterwards.
After six months, he was seen again for the first time since PVL closure when he was re-admitted to hospital for new symptoms of heart failure. We found a new paravalvular leak with severe aortic regurgitation located next to the previously implanted plugs in 3D-TOE. Exact sizing of the new paravalvular defect was impossible in 3D-TOE due to multiple artefacts caused by the previously implanted plugs interfering with the defect. Because of high perioperative risk, the local Heart Team again favoured an intervention-based paravalvular leak closure over a surgical approach. Again, endocarditis was excluded as the underlying cause for the new paravalvular leak. In March 2015, another AVP III (12×5 mm) was successfully implanted into the new paravalvular dehiscence by using a retrograde femoral vascular access even without an exact sizing of the defect in 3D-TOE (Figure 7). Hereafter, a significant reduction in aortic regurgitation from grade III-IV to grade II could again be seen. Several additional attempts to implant another 8×3 mm AVP into the dehiscence and thereby further diminish the residual regurgitation failed. However, even the moderate reduction in aortic regurgitation achieved again led to a rapid clinical stabilisation of our patient. A few days after interventional PVL closure, our patient was discharged from hospital in a markedly improved and stabilised cardiac condition. Three months after the procedure, he is still well without any need for hospital re-admission due to cardiac symptoms.
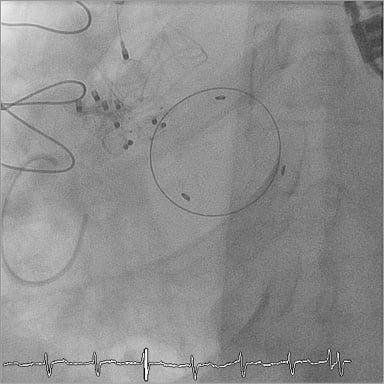
Figure 7. Implantation of the final AMPLATZER Vascular Plug III device (12×5 mm) into the new paravalvular dehiscence next to the previously implanted plug devices.
Pathophysiological causes for re-occurrence of PVL after initial successful interventional closure are speculative for they are not yet known or published. Constant mechanical stress and tractive forces having an impact on the perivalvular tissue on the one hand and further degeneration of perivalvular tissue on the other may play a role in the occurrence of new PVLs. A possible impact on the occurrence of new PVLs by further mechanical irritation of the tissue surrounding the PVL by previously implanted plug devices and incomplete closure of the PVL (as in our case) is also speculative.
To date, clinical data on the long-term follow-up of patients treated by interventional closure of PVL are very limited1,9-11. Unfortunately, at present, all reports including a follow-up give no particular information on the need for reintervention for new paravalvular defects after initial successful interventional therapy1,8-11. According to the case presented here, we presume that a clinically relevant percentage of patients with new paravalvular leaks after successful interventional closure exists in clinical practice. Therefore, regular follow-up visits should be performed after these complex interventional procedures. We recommend regular transthoracic echocardiography, especially during the first months after interventional closure of PVL. In case of clinical or echocardiographical signs of new significant paravalvular regurgitation, 3D-TOE should be performed. Furthermore, all patients treated by catheter-based closure of PVL should be enrolled into a clinical registry to gain data on the safety and efficacy - especially at long-term follow-up - in clinical practice.
This clinical case shows the importance of closely monitoring patients even after initially successful complex interventional procedures, such as catheter-based closure of a paravalvular leak.
Conflict of interest statement
The authors have no conflicts of interest to declare.

