Abstract
Aims: Transcatheter techniques can theoretically be applied to the treatment of para-annular ring (PAR) leaks. Little is known about their potential application and resultant complications in such cases. We describe the first-in-man percutaneous transapical-transseptal Melody valve-in-ring (ViR) implantation after a complication from percutaneous PAR leak closure.
Methods and results: A 49-year-old woman, at high operative risk, presented with congestive heart failure secondary to severe para-ring/extravalvular regurgitation two months after bypass surgery and mitral ring annuloplasty. Successful percutaneous closure of the leak was performed using an AMPLATZER Vascular Plug IV. One month later, she developed haemolysis with severe PAR regurgitation, through and around the device. After device retrieval and placement of an AMPLATZER Muscular VSD occluder, the patient developed severe intravalvular regurgitation. Completely percutaneous, transseptal delivery of a Melody ViR was performed over a transapical-transseptal, arteriovenous rail. Echocardiography revealed trivial residual regurgitation through the implanted valve with mild transvalvular gradients.
Conclusions: Percutaneous closure of mitral PAR leaks after ring annuloplasty in the high-risk patient is feasible (proof-of-concept), particularly when the leak is para-ring/extravalvular. Potential complications include severe intravalvular mitral regurgitation caused by disruption of the mitral apparatus and/or ring deformation during device deployment, which can be successfully treated via percutaneous transapical-transseptal ViR implantation.
Introduction
Mitral valve repair, which includes the implantation of a mitral annuloplasty ring in the majority of cases, is the preferred treatment option when feasible in patients with mitral regurgitation (MR)1. However, repair failure with significant intravalvular and/or para-annular ring regurgitation (PAR) may occur2,3. The gold-standard treatment for severe intravalvular MR is surgery to repair or replace the valve; however, morbidity and mortality rates cannot be ignored and some patients are poor surgical candidates. Alternatives include MitraClip (Abbott Laboratories, Abbott Park, IL, USA) implantation in patients with large annuloplasty rings and preserved leaflet mobility, and valve-in-ring (ViR) procedure in patients with complete rings or incomplete rings protected by surgical aortic valve prostheses. Isolated case reports and small case series have reported the feasibility of transcatheter mitral ViR implantation and the short-term clinical and haemodynamic improvement with failed repair4-9.
Para-annular ring regurgitation can be further divided into para-ring/intravalvular and para-ring/extravalvular types (Figure 1), the former being caused by partial dehiscence of the prosthetic ring from the mitral annulus. Dehiscence is a contraindication to ViR procedure, and percutaneous options for closure using Amplatzer devices (St. Jude Medical, Minneapolis, MN, USA), similar to surgical prosthetic paravalvular leaks, are not possible due to concerns about damage of the native mitral valve leaflet9. Para-ring/extravalvular regurgitation, on the other hand, occurs from tearing of mitral annular tissue without dehiscence of the ring from the annulus or leaflet base. Little is known about percutaneous options for PAR closure and their resultant complications. We describe the first-in-man percutaneous transapical-transseptal Melody ViR implantation after a complication from percutaneous PAR leak closure.
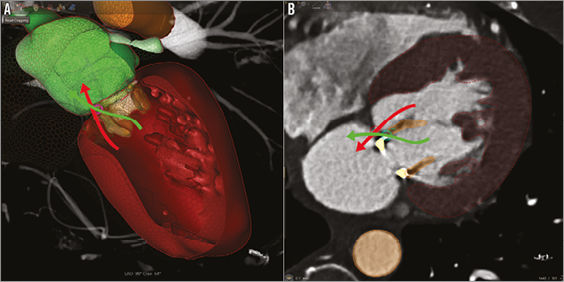
Figure 1. Diagrammatic representation of para-ring types. 3D volume-rendered CTA reconstruction (A) and axial CTA view (B) reveal the two types of para-annular ring regurgitation (PAR): para-ring/intravalvular (green arrow) and para-ring/extravalvular (red arrow). The former identifies flow through the valve but around the ring and the latter around both the valve and the ring. Para-ring/intravalvular type is caused by partial dehiscence of the prosthetic ring from the mitral annulus. Our case involves closure of a para-ring/extravalvular leak. Left atrium: green; left ventricle: red.
Methods and results
PATIENT CLINICAL HISTORY AND INITIAL PRESENTATION
A 49-year-old woman with a medical history of uncontrolled diabetes mellitus, triple-vessel coronary artery disease and severe mitral regurgitation underwent four-vessel CABG and placement of a 26 mm Carpentier-Edwards Physio annuloplasty ring (Edwards Lifesciences, Irvine, CA, USA) two months prior to presentation. Her postoperative course was complicated by two admissions for congestive heart failure, and a transoesophageal echocardiogram (TEE) revealed severe mitral PAR at the one o’clock location, no intravalvular regurgitation, a mean antegrade transmitral gradient of 5 mmHg, and preserved ejection fraction (Figure 2A, Figure 2B). Laboratory values were significant with an NT-proBNP of 2,178 pg/mL, haemoglobin of 11.7 gm/dL, and LDH 247 U/L. Computed tomographic angiography (CTA) with 3D/4D reconstruction showed the para-ring/extravalvular location of the PR leak, measuring 5×6 mm, and stability of the annuloplasty ring, measuring 15×24 mm internally (manufacturer’s original sizing 16.6×24.9 mm) (Figure 2C, Figure 2D). The patient was deemed high risk for re-do mitral valve replacement with an STS-PROM of 8% and, given the size and extravalvular location of the leak, a decision was made by the multidisciplinary team to proceed with percutaneous PAR leak closure. Written informed consent was obtained.
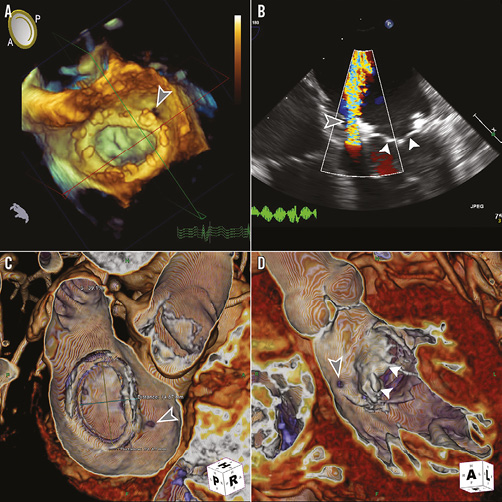
Figure 2. Pre-procedural transoesophageal echocardiography (TEE) and computed tomographic angiography (CTA). A) 3D TEE shows the complete annuloplasty ring with the para-annular ring leak identified at the 1 o’clock location in the surgeon’s view (grey arrowhead). B) 2D TEE with colour Doppler reveals the large regurgitant jet around the annuloplasty ring with the native mitral valve leaflets well visualised (white arrowheads). C) and D) CTA with 3D/4D reconstruction (TeraRecon, San Mateo, CA, USA) confirms the location of leak around the ring and native leaflets and is used to size the ring’s internal dimensions.
FIRST PROCEDURE
CTA-fluoroscopy and TEE-fluoroscopy fusion imaging were utilised for procedural planning and guidance (HeartNavigator and EchoNavigator; Philips Healthcare, Best, The Netherlands). Transseptal puncture was performed with an 8 Fr Fast-Cath™ 63 cm sheath (St. Jude Medical) and a 71 cm Brockenbrough needle. Heparin was administered to achieve an activated clotting time (ACT) >250 sec. A 0.035” Angled Glidewire (Terumo Medical Corporation, Somerset, NJ, USA) and a 5 Fr internal mammary artery (IMA) catheter were used to cross the PR leak, and the sheath was advanced into the left ventricle (LV) over the catheter. A 0.025” guidewire (Toray International America Inc., New York, NY, USA) was advanced into the LV as a safety wire, and an 8 mm AMPLATZER Vascular Plug (AVP) IV (St. Jude Medical) was positioned and deployed across the leak with trivial residual regurgitation through the device (Figure 3).
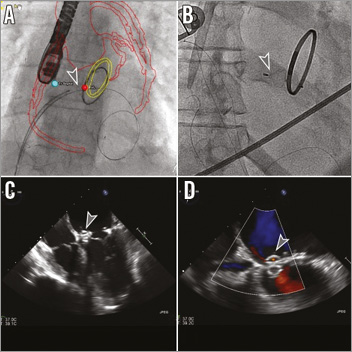
Figure 3. Percutaneous para-annular ring leak closure. Using CTA-fluoroscopy fusion imaging for guidance, an outline of the aorta with coronary arteries and saphenous vein bypass grafts (red) and mitral ring (yellow) can be visualised. Sites of transseptal puncture and para-ring leak are identified by blue and red dots, respectively. An AMPLATZER Vascular Plug IV (AVP IV; grey arrowhead) (St. Jude Medical, Minneapolis, MN, USA) was positioned (A) and deployed (B) within the para-ring leak, with a 0.025” guidewire (Toray, New York, NY, USA) remaining in the left ventricle. TEE 4-chamber view (C) shows the AVP IV with trivial residual flow through the device on colour Doppler (D).
RE-PRESENTATION WITH SEVERE HAEMOLYSIS
The patient re-presented one month later with worsening of dyspnoea, NYHA III and generalised weakness. She was found to have severe haemolytic anaemia with a haemoglobin level of 6.5 mg/dL, haptoglobin <8.0 mg/dL, and LDH 3,418 U/L and acute renal failure with a creatinine level of 3.9 ml/dL. She received a total of 6U of packed red blood cells. Repeat TEE demonstrated severe mitral regurgitation through and around the previously placed AVP IV device, borderline ejection fraction 50%, and pulmonary artery systolic pressure of 60 mmHg (Figure 4A). The recalculated STS-PROM was 33% and, following further discussion with the multidisciplinary team, the decision was made to remove the AVP IV and to place an alternative Amplatzer device.
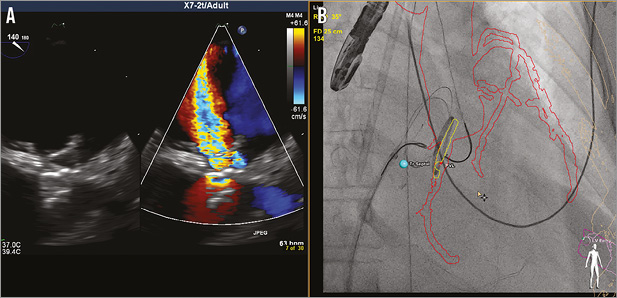
Figure 4. Re-presentation with severe haemolytic anaemia. A) TEE performed upon re-presentation demonstrated the AVP IV still within the para-ring leak, but with severe mitral regurgitation through and around the device as noted on colour Doppler. B) The AVP IV was retrieved using two Amplatz GooseNeck snares (ev3, Inc./Covidien, Plymouth, MN, USA) through JR 4.0 guide catheters from both transseptal and retrograde transaortic approaches.
SECOND PROCEDURE
Transseptal puncture was again performed using a similar technique with the 8 Fr Mullins sheath (Medtronic, Minneapolis, MN, USA) exchanged for an 8 Fr TorqVue™ sheath (St. Jude Medical). Heparin was administered to achieve an ACT >250 sec. A 6 Fr sheath was inserted into the right common femoral artery and, from both transseptal and retrograde transaortic approaches, the AVP IV was retrieved using two 20 mm Amplatz GooseNeck snares (ev3, Inc./Covidien, Plymouth, MN, USA) through JR 4.0 guide catheters (Figure 4B). Dual access retrieval was intended to elongate the device prior to removal to reduce the potential for damage to the mitral apparatus. Access was maintained across the PAR leak with the advancement of the TorqVue sheath and placement of an 8 mm AMPLATZER Muscular VSD device (St. Jude Medical) using the same 0.025” safety guidewire (Figure 5A). Prior to release of the device, TEE revealed no residual PR leak, but severe intravalvular regurgitation (Figure 5B, Figure 5C). The patient remained haemodynamically stable; however, she was noted to have increasing oxygen requirements on the ventilator.
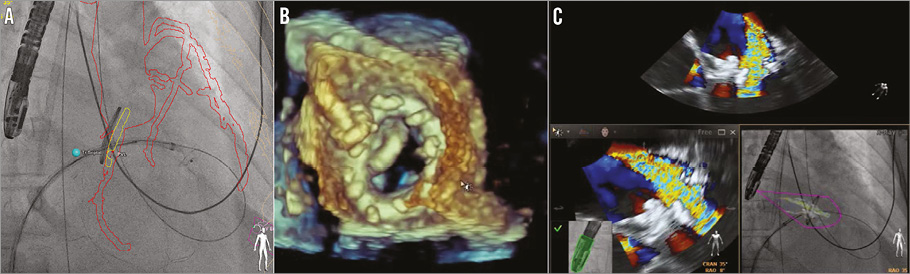
Figure 5. Complicated re-attempt at percutaneous para-annular ring leak closure. A) Using CTA-fluoroscopy fusion imaging (HeartNavigator; Philips Healthcare, Best, The Netherlands), an 8 mm AMPLATZER Muscular VSD device (mVSD) (St. Jude Medical) can be visualised being pulled back into the para-ring (PAR) leak, with the distal disc exposed from the sheath. B) 3D transoesophageal echocardiography (TEE) revealed appropriate positioning of the mVSD. C) TEE with colour Doppler showed no residual PAR leak, but severe intravalvular regurgitation (upper and lower left panels). TEE colour Doppler overlayed onto fluorosocopy using TEE-fluoroscopy fusion imaging (EchoNavigator; Philips Healthcare) confirmed the location of the intravalvular leak without regurgitation at the site of the attached PAR device.
Given wide-open MR and the corrected PAR leak, mitral ViR using a Melody valve (Medtronic) was discussed as bail-out and considered the best option given the patient’s high risk as a surgical candidate (Figure 6). The technique of a completely percutaneous transseptal-transapical approach, described previously, is accomplished by utilising an arteriovenous (AV) rail10. This approach allows for venous valve delivery and, we believe, the necessary coaxial and precise positioning required for ViR implantation. The SAPIEN valve (Edwards Lifesciences, Irvine, CA, USA) was not approved for use in the USA during the time of the intervention.
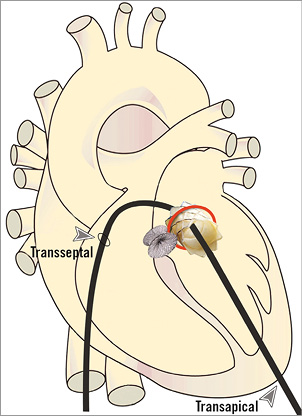
Figure 6. Diagram of intended percutaneous transapical-transseptal Melody valve-in-ring (ViR) implantation. After complication from para-ring leak closure with severe intravalvular mitral regurgitation, the decision was made to proceed with mitral ViR using a completely percutaneous approach. With both percutaneous transseptal and transapical access, an arteriovenous rail is created over which transseptal delivery of a Melody valve can be performed. This drawing depicts the planned mitral Melody ViR implantation with a previously treated para-ring leak using an AMPLATZER Muscular VSD occluder.
A percutaneous transapical puncture was performed using fusion-imaging guidance, with a 6 Fr 13 cm radial sheath (Cook Medical, Bloomington, IN, USA) inserted into the left ventricle11,12. A 7 Fr balloon-tipped catheter was advanced from the antegrade transseptal approach across the mitral and aortic valves into the ascending aorta to prevent entanglement within the native mitral apparatus. A 0.035” Angled Stiff Glidewire (Terumo Medical Corporation) was advanced through the balloon-tipped catheter and exteriorised transapically in the aorta using an 18-30 mm EN Snare® (Merit Medical Systems, Inc., South Jordan, UT, USA), creating an AV rail. Next, the 8 Fr TorqVue venous system was upsized to a 24 Fr DrySeal sheath (Gore Medical, Wilmington, DE, USA) and balloon interatrial septostomy was performed using a 6.0×40 mm peripheral balloon, inflated to nominal pressures.
The Melody valve (Medtronic) was prepared per protocol, mounted however onto a 24 mm BiB balloon (NuMED, Hopkinton, NY, USA) inserted within an Ensemble delivery system (Medtronic). The system was advanced through the DrySeal into the left atrium. The valve was unsheathed and the inner balloon inflated first to allow for fine-tune positioning, followed by final outer balloon inflation (Figure 7). The optimal angle for deployment was determined based on fusion imaging such that the fluoroscopy plane was perpendicular to the ring. Fluoroscopy and TEE were used to confirm Melody valve positioning with the depth intended to be 40:60 (atrium/ventricle) to avoid LV outflow tract obstruction with a too ventricular or angulated implant. Transapical access was closed using a 6/4 mm AMPLATZER Duct Occluder (St. Jude Medical).
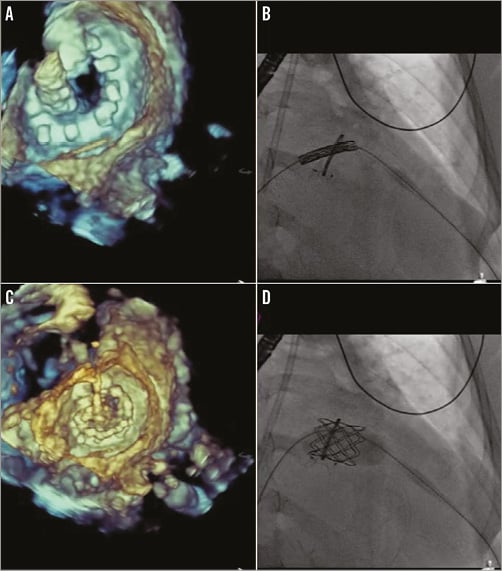
Figure 7. Percutaneous transapical-transseptal Melody valve-in-ring (ViR) implantation. After transseptal and transapical access and creation of an arteriovenous (AV) rail, the Melody valve (Medtronic) was mounted onto a 24 mm BiB balloon (NuMED) within an Ensemble (Medtronic) delivery system. A) 3D transoesophageal echocardiography (TEE) can confirm the position of the valve across the mitral ring. B) With the optimal fluoroscopy angle determined such that the ring is perpendicular to the c-arm, fine-tune positioning of the valve occurs using tension on the AV rail and adjustments of both the delivery system and the transapical sheath. The inner balloon is first inflated; final outer balloon inflation is performed and confirmation of 40:60 (atrium/ventricle) positioning can be noted on both TEE (C) and fluoroscopy (D).
FOLLOW-UP
Post-procedural TEE and transthoracic echocardiography (TTE) showed trivial mitral regurgitation between the ring and Melody valve, an antegrade mitral gradient of 5 mmHg, reduced pulmonary artery systolic pressure of 40 mmHg, normal ejection fraction, and no evidence of LV outflow tract gradient (Figure 8). At one-month follow-up, the patient reported improved dyspnoea (NYHA I), and laboratory data showed resolved haemolysis. She remained on haemodialysis, but was making urine.
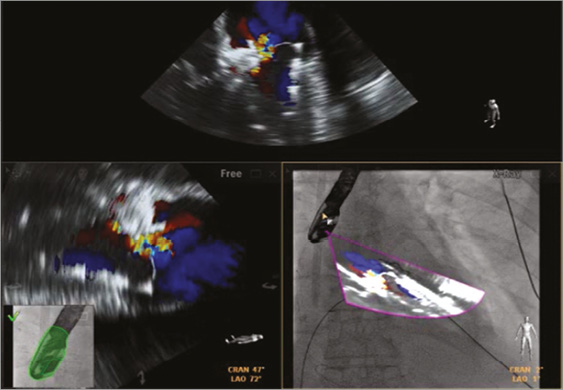
Figure 8. Post-procedural imaging after Melody valve-in-ring (ViR) implantation. Using fusion imaging with the EchoNavigator system (Philips Healthcare), transoesophageal echocardiography (TEE) with colour Doppler revealed successful para-ring closure with an AMPLATZER Muscular VSD device and Melody ViR implantation. Trivial residual regurgitation was noted through the valve, in the setting of the railed arteriovenous wire, and no residual para-ring regurgitation. TEE overlayed onto live fluoroscopy showed the fused TEE image in the same angle as the x-ray camera (bottom right panel).
Discussion
Percutaneous closure of prosthetic paravalvular leaks has been well reported with a multitude of transcatheter devices used off-label for this purpose13-16. However, percutaneous PAR leak closure after a failed mitral annuloplasty has, to the best of our knowledge, never been described. The location of the leak, the type of ring, and technique of closure should be considered with a contingency plan should injury to the native mitral apparatus occur. Para-ring/extravalvular regurgitation can potentially be amenable to percutaneous closure as this occurs from tearing of mitral annular tissue, without ring dehiscence. Nonetheless, there remains limited experience regarding this patient population, and a decision for treatment should be made in a multidisciplinary fashion. The closure chosen should entail minimal protrusion into the LV cavity since this can cause repetitive leaflet damage as the valve opens and closes. An AVP IV was initially chosen because of its small profile and lack of retention discs, allowing for minimal contact with the valve.
Over a short follow-up period, it was clear that the degree of regurgitation worsened, both around and through the device. A decision was made to replace the device with an occluder that had interwoven polyester for more complete sealing, in this case a Muscular VSD. Unfortunately, during positioning, severe intravalvular regurgitation was noted. As the distal retention disc was exposed within the LV and while being pulled into the PAR leak, it is possible that the chordae tendinae of the mitral leaflet were disrupted. In addition, the patient had a CE Physio annuloplasty ring. This ring is composed of layers of Elgiloy and plastic strips with a sewing ring margin that consists of silicone rubber covered by polyester fabric. The posterior flexibility of the ring allows for physiologic contractility of the mitral annulus during systole. The deployment of a more rigid occluder device, such as the Muscular VSD (mVSD), can potentially cause ring deformity and lead to valvular regurgitation. Using this technique may be more effective in annuloplasty rings with a rigid core.
This unique complication of para-ring leak closure can potentially be managed with the use of alternative devices. The AVP II has finer nitinol mesh which may provide less radial force and conform better to the leak without ring deformation. However, if leaflet disruption is noted, surgical correction has traditionally been the only treatment option available. Recent experience with ViR implantation has provided an alternative to surgery in high-risk patients with mitral valve dysfunction following annuloplasty5-9. The most commonly used transcatheter prosthesis is the SAPIEN XT valve (Edwards Lifesciences, Irvine, CA, USA), due to the availability of different sizes and the known durability under left-sided heart pressures17. The Melody valve, on the other hand, has been successfully implanted within multiple types of mitral annuloplasty rings in animal models via percutaneous transfemoral venous and transatrial approaches18,19. Although the long-term function of this valve when subjected to systemic pressures is largely unknown, increasing follow-up data are encouraging20,21. In this case, the Melody valve was mounted onto a 24 mm BiB balloon to expand the valve to its maximal recommended size, while being loaded into an Ensemble system ensuring lower profile delivery (Melody: 24 Fr, commercial US SAPIEN: 28 Fr). It was also the only commercially available valve in the USA at the time of implantation.
Typical delivery approaches for the ViR procedure described include percutaneous transseptal and open surgical transapical. However, early mitral valve-in-valve (ViV) experience has reported unsuccessful deployment with percutaneous transseptal or direct transatrial approaches, due to non-coaxial and too ventricular positioning22. The technique of the percutaneous transapical-transseptal approach, while utilising an AV rail, may provide a more precise method of venous delivery for ViR, while avoiding open surgical exposure. Due to the Melody’s covered stent design, precise implantation is important, as too ventricular positioning can lead not only to valve embolisation, but also to outflow tract obstruction via displacement of the subvalvular mitral apparatus and orientation of the prosthesis towards the outflow tract. On the other hand, too atrial positioning may result in pulmonary vein stenosis physiology, more likely in patients with a small or normal-sized left atrium.
Conclusion
Re-do mitral surgery (replacement or re-repair) has been the gold standard for patients with recurrent mitral regurgitation after valve repair. Percutaneous options in the high-risk patient are now feasible (proof-of-concept) for the treatment of para-ring leaks, particularly when the regurgitation is para-ring/extravalvular. The potential complication of severe intravalvular MR caused by disruption of the mitral apparatus during device deployment or ring deformation can be successfully treated via completely percutaneous transapical-transseptal ViR implantation.
| Impact on daily practice Transcatheter techniques can be applied for para-annular ring (PAR) leak closure in the appropriate patient. Understanding potential complications, most importantly disruption of the mitral apparatus and/or ring deformation, is essential, as is a non-surgical rescue therapy. Percutaneous ViR implantation can be performed successfully and the steps considered prior to performing PAR leak closure. |
Conflict of interest statement
C. Kliger has received a speaking honorarium from St. Jude Medical, and Philips Healthcare. G. Fontana is a consultant for St. Jude Medical, Medtronic, Sorin Medical, Entourage Medical Technologies, Edwards Lifesciences, and Paieon Inc., has received speaking honoraria from St. Jude Medical, Medtronic, Edwards Lifesciences, and Sorin Medical, and has stock/stock options in Entourage Medical Technologies. I. Kronzon is a consultant for Philips Healthcare. C. Ruiz is a consultant for Valtech, St. Jude Medical, and Sorin Medical, receives research grants from Philips Healthcare, and has financial interest in Vascular Therapies, MitrAssist, Entourage, and BioInspire. The other authors have no conflicts of interest to declare.

