Abstract
Aims: A “valve-in-ring” (ViR) procedure involves the transcatheter implant of a valved stent in a prosthetic mitral ring. The presence of a partial dehiscence of the prosthetic ring is a major contraindication for a ViR due to inefficacious sealing. We describe an alternative method of ViR implant to achieve proper valve sealing in the case of ring dehiscence.
Methods and results: A 76-year-old male patient suffered from severe central mitral regurgitation due to annuloplasty ring dehiscence and leaflet tethering. ECG-gated multidetector computed tomography was used for preoperative planning. Standard transapical access was gained through a minimally invasive left thoracotomy in the 5th intercostal space. A customised Melody® valve with two PTFE sutures fixed to the apex was used. The intervention was performed without complications, the patient recovered well, and transthoracic echo revealed no mitral regurgitation through the implanted valve with a transvalvular gradient of 4 mmHg.
Conclusions: The implantation of a long covered stent such as the Melody valve allows successful sealing following a ViR even in case of partially detached annuloplasty rings. This procedure is a proof of concept that proper sealing can be achieved at the leaflet level without the use of radial force at the annular level.
Abbreviations
CT: computed tomography
EDVI: end-diastolic volume index
LAVI: left atrial volume index
LVEDP: left ventricular end-diastolic pressure
MR: mitral valve regurgitation
NYHA: New York Heart Association
ViR: valve-in-ring
Introduction
A “valve-in-ring” (ViR) procedure involves the transcatheter implant of a valved stent in a prosthetic mitral ring. The main indication for a ViR is recurrent mitral valve regurgitation (MR) following open heart mitral valve repair with a prosthetic ring1. The most commonly used prosthesis for ViR procedures is the balloon-expandable SAPIEN valve (Edwards Lifesciences, Irvine, CA, USA), while the Melody® valve (Medtronic, Minneapolis, MN, USA) has been used less frequently due to size restrictions, although preclinical studies have demonstrated the feasibility of the implant of a bovine vein conduit in a surgical mitral ring2-4.
In a ViR implant, the implanted surgical ring provides a secure landing structure for the transcatheter valve. A complete apposition of the transcatheter valve along the ring is required to achieve haemodynamic sealing. As a consequence, the valve should usually be slightly oversized and the ring should ideally be flexible, or at least malleable, to conform to the rounded shape of the transcatheter prosthesis.
The presence of a partial dehiscence of the prosthetic ring is a major contraindication for a ViR procedure since, even in the presence of complete apposition between the valve and the ring, regurgitant flow is allowed to pass through the gap between the ring and the native annulus.
We describe an alternative method of ViR implant to achieve proper valve sealing in case of ring dehiscence.
Methods and results
PATIENT CLINICAL HISTORY AND PRESENTATION
A 76-year-old patient was referred to our hospital in cardiogenic shock with biventricular decompensation and NYHA Class IV with a history of mitral valve repair of a type II lesion with a 34 mm Carpentier-Edwards Classic ring implant 26 years previously. Further diagnoses included a permanent atrial fibrillation, a previous TIA, gout with chronic steroid therapy and steroid-induced diabetes. Transthoracic and transoesophageal echocardiography revealed a severe central mitral regurgitation (vena contracta 7 mm) due to annuloplasty ring dehiscence and leaflet tethering (tenting area 1.7 cm2, tenting height 7 mm) (Figure 1). The left ventricular ejection fraction was 25% with a severely dilated hypokinetic left ventricle (EDVI=101 ml/m2), dilated left atrium (LAVI=46 ml/m2). The coronary angiogram showed a 30% stenosis of the right coronary artery, a normal LVEDP of 12 mmHg and an elevated mean pulmonary pressure of 40 mmHg. The cardiac index was 1.4 l/min/m². Renal function was also impaired due to the low output.
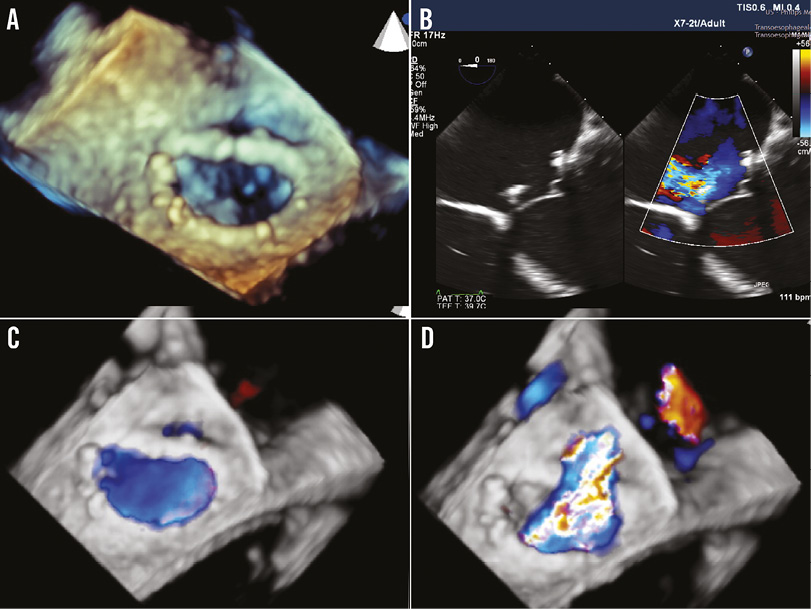
Figure 1. Pre-procedural echo images. A) 3D reconstruction of the ring implant shows the posteromedial dehiscence. B) Colour-compare 4-chamber view showing the dehiscence of the ring. C) 3D full-volume colour view in diastole showing two flows, one internal to the ring and one in the dehiscence. D) 3D full-volume colour view in systole showing the regurgitant jet.
The patient’s condition improved after short-term intravenous therapy with inotropes, diuretics and levosimendan. Due to the high-risk profile, a multidisciplinary Heart Team decision was taken to proceed with a transcatheter ViR transapical implantation using a Melody® valve (Medtronic). Written informed consent from the patient was obtained for the planned procedure.
CT SCAN ANALYSIS
Gated multidetector computed tomography is used as a standard method to assess anatomy, plan the procedure and assist in prosthesis choice (type and size) in most ViR cases. The DICOM file was examined using the OsiriX package (Pixmeo SARL, Bernex, Switzerland). A multiplanar reconstruction was used to measure the ring dimensions independently from the manufacturer sizing characteristics and to size the area of the dehiscence (Figure 2A, Figure 2B). The coaptation depth and the size of the left ventricular outflow tract were analysed to plan the implant depth in order to reach the tip of the anterior leaflet while avoiding subaortic obstruction (Figure 2C, Figure 2D). Finally, CT scan modelling was used to predict the fluoroscopic planes as well as the ideal location of the thoracotomy in order to ensure a coaxial delivery of the devices (Figure 3).
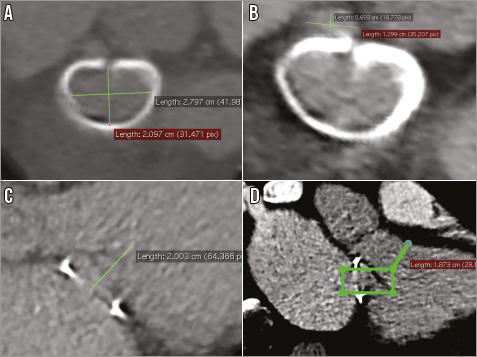
Figure 2. Preoperative multiplanar reconstruction. Measurements of the ring dimensions (A) and the size of the dehiscent area (B). The coaptation depth (C) and the size of the left ventricular outflow tract (D) were analysed to plan the implant depth in order to reach the tip of the anterior leaflet.
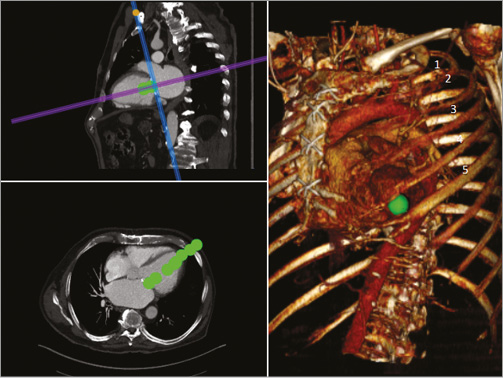
Figure 3. Preoperative CT scan modelling. This was used to predict the fluoroscopic planes and the ideal location of the thoracotomy to ensure a coaxial delivery of the device.
MELODY VALVE PREPARATION
The Melody valve is a bovine jugular vein conduit mounted over a platinum-iridium wire stent involving six rows of circumferential struts. The most important characteristic of its design is the ability to expand beyond the working diameter without shortening to a degree that distorts the valve leaflets. Before mounting it for the intervention, it was rinsed in saline and valve competence was checked with the hydraulic test. Prior to crimping, two PTFE CV-2 sutures (GORE-TEX®; Gore Medical, Flagstaff, AZ, USA) were attached to the most distal portion of the outflow of the stent (Figure 4A). The needles were cut and the two couples of sutures were kept in order using two mosquito clamps (Figure 4B). The PTFE sutures were attached to act as neochordae, to be exteriorised from the apical access and fixed on the apex to protect the device from embolisation (Figure 4C). The valve was then crimped in the usual way, initially around a 5 cc syringe and thereafter on a 25 mm Nucleus balloon (Z-MED; B. Braun Medical, Minneapolis, MN, USA) with the inflow facing the tip of the balloon.
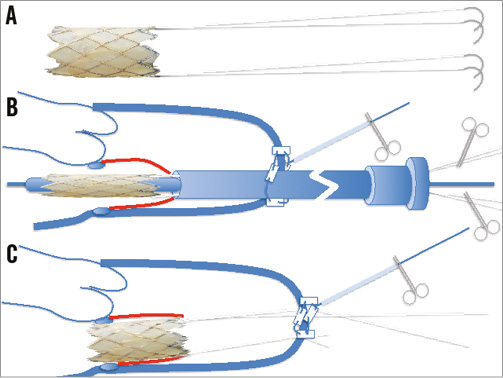
Figure 4. Schematic representation of the customisation of the Melody valve. A) Two double-armed CV-2 sutures are sutured at opposite ends of the outflow of the Melody valve, on the stent struts. B) The sutures are left on the side of the Nucleus balloon shaft, under slight tension to prevent entangling. C) Following the implant, the sutures are exteriorised and tied on the pledgets of the apical purse string to reinforce the implant and to prevent embolisation in the atrium.
PROCEDURE
Arterial and venous access at the groin with 4 Fr sheaths was achieved for eventual rescue circulatory support. According to CT scan prediction (Figure 3), a minimal left thoracotomy, access in the lateral axillary line, in the 5th intercostal space was performed. The pericardium was opened and freed from the adhesions to the epicardium. A muscular area free of epicardial fat was identified and two perpendicular mattress 2-0 polypropylene purse strings with large Teflon strips were applied for bleeding control and subsequent sealing of the apical access. Temporary pacing wires were applied to the epicardial surface adjacent to the purse strings. Following heparin administration, the apex was punctured, a 10 Fr introducer sheath was advanced into the left ventricular apex, and a diagnostic 0.035” J-wire was advanced into the left atrium, through the mitral valve, under TEE and fluoroscopy guidance. The wire pathway was carefully assessed to confirm that the passage was in the middle of the leaflets and not entangled in the subvalvular apparatus. A second wire (0.014” Balance MiddleWeight; Abbott Vascular, Santa Clara, CA USA) was then advanced through the area of the detachment to ensure access to the perivalvular leak in case of residual regurgitation. The diagnostic guidewire was then exchanged for an Amplatz Super Stiff 0.035” wire (ST1) (Boston Scientific, Natick, MA, USA). The 10 Fr sheath was then replaced with a 24 Fr Cook sheath (Cook Medical, Bloomington, IN, USA) to ensure advancement of the bulky “custom-made” implant. The tip of the 24 Fr sheath was shortened to facilitate the exteriorisation of the sutures following the Melody deployment. Prior to Melody implant, acting as a “filler” and to increase friction, a 22 mm CP stent (CP8Z22; NuMED Inc., Hopkinton, NY, USA), crimped over a 25 mm Z-MED balloon (B. Braun Medical) was expanded to cover just the ring structure, to try to minimise any interference with the underlying native leaflets of the valve. The CP stent was inflated until it reached an hour-glass configuration, but trying not to expand the prosthetic mitral ring. Rapid pacing and apnoea were used to land the stent precisely. Following CP stent implantation, the MR appeared slightly increased; however, haemodynamics were unchanged. Under rapid pacing (140 bpm), the 25 mm Nucleus balloon was inflated to implant the valve 20% above the annulus, in the atrium, and 80% in the ventricle, in order to maximise leaflet sealing, whilst not impinging into the outflow tract (Figure 5A). Following Melody implant, MR was immediately reduced from severe to trace. A gentle pull was kept on the PTFE neochordae. The 0.014” wire was then removed. The 24 Fr sheath and the Super Stiff wire were carefully removed, taking care to maintain the appropriate tension on the two neochordae. Following sheath removal, the apical purse string was ligated and the neochordae were fixed to it for embolisation prevention. Valve haemodynamics were considered satisfactory, with a 4 mmHg gradient. The chest was closed in the conventional way and the patient transferred to the intensive care unit.
POSTOPERATIVE FOLLOW-UP AND DISCHARGE ECHO
The patient recovered well on the ICU and was transferred to the ward nine days after surgery. At discharge transthoracic echo, valve function was normal and no MR was detectable.
Discussion
Implant of a ViR is an established alternative to surgery in high-risk or inoperable patients with recurrent mitral valve dysfunction following mitral valve repair with a prosthetic ring1,5-8. Most patients with recurrent MR after ring implantation are affected by functional MR, similar to the patient described here. The mechanism of recurrent regurgitation could be either ongoing ventricular remodelling with increased leaflet tethering9-12, or ring dehiscence, or both. Instead of a ViR procedure, MitraClip implant13 is a potential alternative in patients with larger annuloplasty rings, when leaflet mobility is preserved and the MR jet is not wide. For patients with smaller rings, a ViR remains a better option, since the risk of inducing mitral valve stenosis is probably less. For ViR procedures, an aortic balloon-expandable prosthesis is inserted upside down with either an antegrade (transseptal) or a retrograde (transapical) approach. The most commonly used prosthesis is the SAPIEN XT (Edwards Lifesciences), but the feasibility of a Melody implant has been tested in animal models3,4. The SAPIEN XT valve is more commonly used, mainly since it is available in multiple sizes, up to 29 mm. The Melody can only be expanded up to 24 mm, precluding implant in patients with large rings. In ViR procedures, the annuloplasty ring functions as a retaining structure, as well as the site for valve sealing. Often the implanted SAPIEN valve is not touching the native mitral leaflets, since the tenting height is longer than the stent and the SAPIEN valve seems to work in series with the native mitral valve (Figure 5A, Figure 5B).
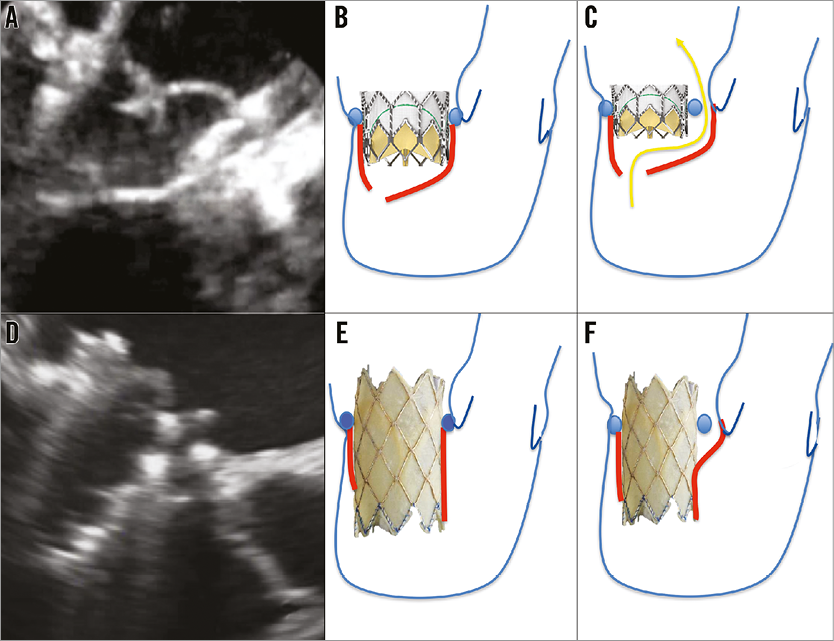
Figure 5. Interaction of the SAPIEN XT valve (A-C) and the Melody valve (D-F) with the mitral valve and the surrounding structures. The SAPIEN valve is not touching the native mitral leaflets, since the tenting height is longer than the stent (A&B). If the ring is partially detached, then sealing at the ring level is ineffective (C). In the case of a longer covered valve stent such as the Melody valve, the native mitral leaflets can seal over the covered stent in systole (D&E), resulting in an effective sealing of the detached ring area (F).
The incidence of ring dehiscence is grossly underreported14-23, and represents a challenge for a ViR procedure: when the ring is partially detached, sealing at the ring level is ineffective (Figure 5C). By implanting a longer covered valve stent such as the Melody valve, the native leaflets can seal over the covered stent in systole (Figure 5D, Figure 5E). As shown in Figure 6A, in diastole, when the native and the stent leaflets are open, there is antegrade flow through the Melody valve as well as through the space between the detached ring and the native annulus. However, in systole, no flow was detected in the area of the dehiscence (Figure 6B) due to effective sealing of the leaflets at the level of the covered stent (Figure 5F). Figure 6C clearly demonstrates that the Melody is not apposed completely on the surgical ring, further clarifying that sealing is not happening at the ring level.
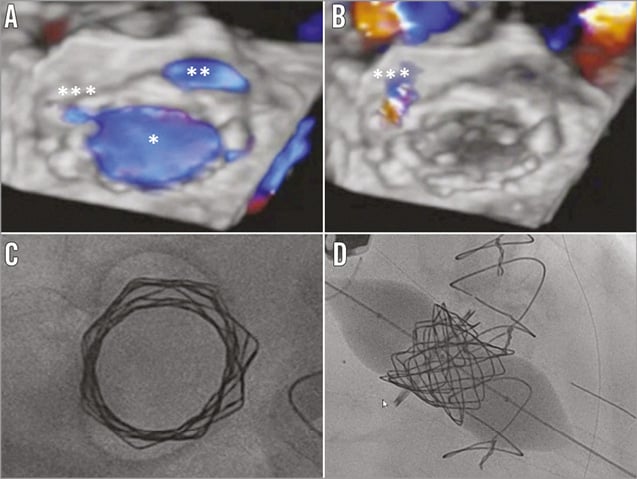
Figure 6. 3D transoesophageal echocardiographic (A & B) and intraoperative fluoroscopic view (C & D). In diastole (A) three orifices are visible: the Melody valve orifice (*), the orifice between the ring and the annulus at the site of annular dehiscence (**), and a third small orifice between the Melody and the prosthetic ring (***). In systole (B) there remains only a minimal perivalvular regurgitation from the last orifice (***), as the result of good Melody function and of the sealing of the native leaflets around the stent. The 25 mm Nucleus balloon creates an hourglass configuration of the Melody after balloon expansion (C & D).
As an alternative to the method here described, a long covered stent with a SAPIEN valve inside it could be used instead. However, in order to prevent massive MR between the covered stent implant and the SAPIEN deployment, the two stents should be crimped one above the other, increasing consistently the profile of the delivery system. Also, a larger covered stent could be more at risk of inducing an obstruction in the LV outflow tract.
The main limitation of the Melody valve in the mitral position is its relatively small size as compared to the SAPIEN valve. In this case, the anterolateral dimension of the ring, as calculated by CT scan, was 21 mm, in close accordance with the manufacturer’s specifications. On the other hand, the intercommissural dimension was well beyond 24 mm, the maximal diameter achievable with a Melody valve. To increase stability, we opted to implant a short CP stent on the surgical ring, in order to increase the thickness and friction at this level. The surgical ring in this particular case was a Carpentier-Edwards Classic, a rigid (but malleable) ring with a peculiar characteristic: the anterior portion of the ring is open. As a consequence, any attempt to oversize the prosthesis would have resulted in a further opening and increase of the anteroposterior dimension of the ring, reducing the radial force resistance to embolisation. As a further safety feature, in order to prevent and eventually correct valve embolisation due to either further ring detachment or to poor radial force at the interface between the Melody and the surgical ring, we created two couples of neochordae with CV-2 e-PTFE sutures (Figure 4). These sutures were kept under tension after valve deployment and until fixed to the transapical purse-string Teflon pledgets. Whether this additional feature is mandatory is impossible to answer at this time; however, it did not increase the complexity of the procedure and provided enough of a safety margin for an innovative first-in-man experience. Transapical implantation of neochordae has been described both as a standalone technique to treat prolapsing leaflets24,25 and as a support for transcatheter mitral valve implantation26.
To enhance stability we also used a 25 mm Nucleus balloon, to achieve an hourglass configuration of the Melody after balloon expansion (Figure 6D). In addition, the use of a Nucleus balloon prevented further expansion of the surgical ring at its mid portion. Theoretically, balloon expansion of the surgical ring could provoke further ring dehiscence, and would also decrease the radial force applied by the Melody valve.
Due to the complex construction (Melody with PTFE sutures), we chose a Cook sheath with an inflatable valve (Cook Medical) to minimise the risk of stent migration during the delivery into the left ventricle. Most importantly, great care was taken to prevent and to rule out entanglement of the delivery wire into the subvalvar apparatus, since its integrity was instrumental for valve sealing. Prior to valve implant, an additional wire was advanced into the perivalvular space of the ring dehiscence, in order eventually to deliver an occluder device in case of inefficacious sealing. Plug implant with an Amplatzer device (St. Jude Medical, St. Paul, MN, USA) in combination with ViR could be considered as an alternative; however, its feasibility is unclear due to the unfavourable geometry of a ring dehiscence (as compared to a valve detachment) for proper plug seating and sealing.
In this particular case, a transapical route was chosen. The transseptal approach has been successfully adopted to deliver ViR; however, the transapical approach offers a number of advantages, including a coaxial orientation of the delivery system, a greater precision of the implant, and in our case also the opportunity of delivering, exteriorising and fixing neochordae on the transapical purse string.
In conclusion, conventional ViR procedures require proper fixing of the surgical ring, in order to achieve sealing. Partial ring dehiscence has been considered a relative or absolute contraindication due to the risk of inadequate sealing and the potential for further ring detachment. Using a covered valved stent inside the native leaflets to achieve sealing is an effective alternative. This solution could also be used in case of rigid rings, when the surgical annuloplasty device is unable to conform to a round configuration. Also, this procedure is a proof of concept that proper sealing can be achieved at the leaflet level without annular apposition, a concept underlying most novel technologies for transcatheter mitral valve implantation26,27.
| Impact on daily practice A partial mitral annuloplasty ring dehiscence is considered to be a contraindication for a conventional valve-in-ring procedure because it risks inadequate sealing or even results in further ring detachment. This procedure is a proof of concept that proper sealing can be achieved with a covered valved stent (Melody valve) inside the native leaflets and that an interventional approach is a feasible alternative for re-operation in a high-risk patient. |
Conflict of interest statement
F. Maisano is a consultant for Abbott, Medtronic, St. Jude, and Valtech Cardio, and is a co-founder of 4Tech and AFfix Medical. F. Nietlispach is a consultant for Edwards Lifesciences and St. Jude Medical. The other authors have no conflicts of interest to declare.

