Abstract
Research and development of percutaneous therapy for mitral regurgitation (MR) began over a decade ago with concepts based on coronary sinus annuloplasty which had the potential to mimic established surgical procedures. While at the present time leaflet repair with the MitraClip has become the most frequently used device approach, several devices that accomplish direct annuloplasty have recently made important advances. Still in their early stages, three direct annuloplasty devices have been used in early human clinical experience, and other methods have been tested in preclinical models. As annuloplasty itself has been the mainstay of surgery for functional MR (FMR) for decades, the prospect of transcatheter direct annuloplasty is, by its nature, more attractive and the devices involved more closely mimic surgery. As these procedures and devices are still evolving, their future role needs to be further defined and established.
Introduction
Percutaneous therapy for mitral regurgitation (MR) had the first concepts in early stages of development over a decade ago1. The initial ideas were based on coronary sinus annuloplasty since that seemed to be relatively simple from a technical standpoint, and have the potential to mimic an established surgical procedure2. Over the last decade, the field of catheter intervention has advanced significantly3. One coronary sinus annuloplasty device has received CE approval4, but several have fallen by the wayside5 and others remain in the earliest stages6. Leaflet repair with the MitraClip (Abbott Vascular, Santa Clara, CA, USA) has become the most frequently used device approach with both FDA and CE approval7,8. In parallel, several devices that accomplish direct annuloplasty have made important strides in the last two or three years. Transcatheter direct annuloplasty is promising because annuloplasty has been the mainstay of surgery for functional MR (FMR) for decades, and devices that more closely mimic surgery are inherently more attractive. Three direct annuloplasty devices have been used in early human clinical experience, and other methods have been tested in preclinical models.
Cardioband™
Among the various annuloplasty devices, the Cardioband™ (Valtech Cardio, Or Yehuda, Israel) most closely resembles a surgical annuloplasty ring (Figure 1)9. The procedure is performed using transseptal puncture for left atrial access. The device is implanted directly into the left atrial side of the mitral annulus. The Cardioband has completed enrolment in a CE approval trail (ClinicalTrials.gov identifier NCT01841554). The inclusion criteria are moderate to severe functional MR, NYHA Class II-IV despite optimal medical therapy, including CRT if indicated, LVEF ≥25%, LVEDD ≤65 mm, the subject is at a high risk to undergo MV surgery (as assessed by a surgeon and a cardiologist, at the site), and transseptal catheterisation and femoral vein access are determined to be feasible. The Valtech Cardioband is delivered via transseptal atrial access. The ring is implanted directly in the atrial side of the mitral annulus. The first anchor is deployed in the lateral commissure and then the ring is extruded from a delivery catheter in small segments, each in turn anchored by a screw mechanism encompassing the posterior annulus until the last anchor is implanted in the medial commissure. The band can be tensioned to reduce the annular circumference and reduce the degree of MR. Results at this point include patients with FMR treated between February 2013 and July 2015. Forty-five high-risk patients with significant FMR were enrolled at six sites in Europe10. After a Heart Team evaluation, all patients were screened by echocardiography to assess eligibility. The mean age was 71±8 years, thirty-four patients were male (76%). Mean logistic EuroSCORE was 17±12%. At baseline, 87% of patients were in NYHA Class III-IV with mean EF of 32±11% (15%-59%). Device implantation was feasible in all patients (100%). MR reduction to ≤1+ was achieved in 84% of the patients (38/45) intra-procedure. After cinching of the device, remodelling of the mitral annulus with an average of 20% reduction of the septo-lateral diameter was observed (from 36±5 mm to 29±6 mm; p<0.01). No procedural mortality occurred and 30-day mortality was 4.4% (adjudicated as unrelated to the device). At six-month follow-up, 82% of patients were in NYHA Class I-II (n=22, p<0.05) with significant improvement in quality of life (MLWHFQ from 38 to 18; p<0.05; n=21) and 86% of patients had MR ≤2+ (n=22). At 12-month follow-up, 94% of patients had MR ≤2+ (n=17), 68% of patients were in NYHA Class I-II (n=18, p<0.05) with significant improvement in quality of life (MLWHFQ from 35 to 19; p<0.05; n=16). These results suggest that transseptal direct annuloplasty with an adjustable “surgical-like” ring is feasible, with good safety (safety performance similar to other transcatheter mitral procedures). Effective reduction in MR severity is observed in most patients related to a significant septolateral dimension reduction. MR reduction is stable and consistent up to 12 months, with significant clinical benefit.
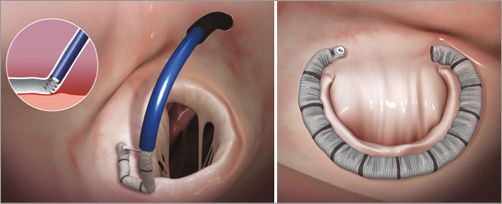
Figure 1. The left panel shows a transseptal guide catheter delivering the annuloplasty ring in segments. Each segment is sequentially anchored into the annulus. The right panel shows the final annuloplasty ring encircling the posterior leaflet. Images used with permission from Valtech, Inc.
Mitralign®
The Mitralign® Percutaneous Annuloplasty System (Mitralign Inc., Tewksbury, MA, USA) is a transventricular device that places pairs of pledgets on either side of the mitral orifice. Each pair is tensioned to reduce the mitral circumference (Figure 2). The procedure is performed via retrograde left ventricular (LV) catheterisation. A guiding catheter is placed into the posterior mitral annular space. Radiofrequency wires are used to traverse the annular tissue from the LV to the left atrial side of the annulus. The wires are used to pass unique pledgeted sutures through the annulus. The pledgets are introduced in pairs using a “bident” device that spaces each pair of pledgets at a predetermined distance apart. The pledgets can then be drawn together and locked into place, which reduces the distance between them and thus the circumference of the mitral annulus (Figure 2, inset). Two or more pledget pairs may be used to achieve optimal reduction of the annular circumference with the two goals of reversing the LV remodelling and improving symptoms. In comparison to other devices, the Mitralign platform has arguably the smallest device footprint due to the small size of the pledgets and the sutures that are ultimately left behind as permanent implants.
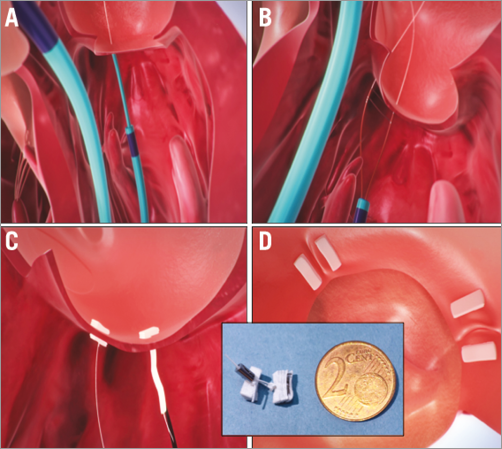
Figure 2. Mitralign annular plication: A) Retrograde guide catheter in the LV, with the distal catheter tip under the mitral annulus, behind the posterior leaflet (arrow). A wire has been passed from the left ventricle (LV) through the annulus and into the left atrium (LA). A second wire is then introduced with a bident catheter (B). Pledgets are passed over the wires (C). Two pairs of wires are used to place pledgets near both commissures (D). The pledgets are drawn together (inset) to decrease the mitral annular circumference. Images used with permission from Mitralign, Inc.
This device has completed enrolment in a CE approval trial (ClinicalTrials.gov identifier NCT01740583)11. The inclusion criteria include NYHA Class II-IV, structurally normal mitral valve, at least Grade 2 mitral regurgitation, LVEF 20%-45%, and LV end-diastolic diameter 5.0-7.5 cm. The CE approval trial included 51 patients with a mean age of 68.5±11 years, mean LVEF 32.7%±8.5%, with about half NYHA Class III or IV. Thirty-day mortality was 7.8% and 30-day stroke rate 5.9%. Six-month mortality was 12.2%. After six months, there were significant improvements in annular dimensions, MR measures, and LV remodelling. Mitral annulus anterior-posterior and septal-lateral diameters decreased by 8% and 6%, or from 3.60±0.4 cm to 3.30±0.3 cm and 3.56±0.4 cm to 3.34±0.4 cm, respectively. Effective regurgitant orifice area (EROA) decreased from 0.34±0.1 cm to 0.29±0.1 cm. LV end-diastolic volume decreased from 186.69±50.8 ml to 164.62±44.2 ml (p<0.01) and LV end-systolic volume decreased from 122.21±42.9 ml to 107.28±33.7 ml (p<0.01). Two pairs of pledgets could be implanted in 80% of cases, and two pledget pairs were obviously more effective at reducing the mitral circumference and measures of MR than a single pair12. In the preliminary results from the CE study, with one pair of pledgets, 39% of patients had ≤2+ MR compared to 63% with ≤2+ MR if two pairs of pledgets were used.
Recently, the Mitralign system has been applied to the tricuspid valve in cases of severe tricuspid regurgitation. The use of pledgeted sutures to plicate the tricuspid posterior annulus is an established surgical approach13. Suture “bicuspidisation” was performed by placement of a pledget-supported mattress suture from the antero-posterior to the posteroseptal commissures along the posterior annulus. Surgical bicuspidisation annuloplasty compared to ring annuloplasty in a group of 237 patients (bicuspidisation in 157 versus ring in 80) was effective at eliminating tricuspid regurgitation at three years postoperatively. The bicuspidisation approach utilising the Mitralign system has now been successfully employed in several patients with severe tricuspid regurgitation14. The percutaneous approach exactly mimics the surgical procedure. The feasibility of the Mitralign system for the tricuspid application has opened the new field of percutaneous treatment for tricuspid valve disease.
Guided Delivery Systems
The GDS Accucinch® System (Guided Delivery Systems, Inc., Santa Clara, CA, USA) implants multiple anchors in the mitral basal annular myocardium. A tether connects the anchors and tension of the catheter diminishes the mitral annular circumference (Figure 3). This approach results in both diminution of the annular circumference and, at the same time, stabilisation or remodelling of the basal LV myocardium. The concept is thus a combination of LV remodelling and annuloplasty.
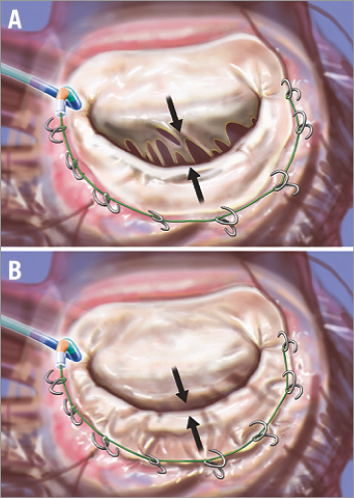
Figure 3. The Guided Delivery Systems Accucinch device is delivered through retrograde catheterisation of the LV (A). The arrows highlight the separation of the leaflet edges, which define the regurgitant orifice. Anchors are placed in the posterior mitral annulus and connected with a “drawstring” to cinch the annular circumference. When the cord is tightened, the basilar myocardium and annulus draw the mitral leaflets together to decrease the regurgitant orifice (B). From reference 3; artwork by Craig Skaggs.
The Guided Delivery Systems Accucinch device places a delivery system below the posterior mitral leaflet on the ventricular side, through which between 10 and 20 nitinol anchors are delivered. These anchors are tethered by a drawstring, which is tensioned to reduce the mitral annulus. Several patients have been treated, but the device is still under development. An incomplete ring approach has some precedent in surgery. Surgical annular plication without placement of an annular ring has demonstrated some efficacy in reducing MR15-17.
Several patients have been treated over the last few years as the system has evolved, but there is no published experience. A Feasibility Study Using the Accucinch System in the Left Ventricular Reshaping of the Mitral Apparatus to Reduce Functional Mitral Regurgitation and Improve Left Ventricular Function Trial (LV RECOVER, ClinicalTrials.gov identifier NCT02153892) has been designed. The inclusion criteria include subjects with severe symptomatic functional LV of ≥3+ secondary to LV remodelling or annular remodelling, as measured in accordance with the current ASE guidelines and suitable for treatment in accordance with the current AHA/ACC guidelines with LVEF ≥20% and ≤40%, stable cardiac medical regimen for heart failure for at least one month and stable NYHA Classification (Class III and above) for at least one month. A second study, Percutaneous Left Ventricular Reshaping to Reduce Functional Mitral Regurgitation and Improve LV Function (LVRESTORESA), is also listed on the ClinicalTrials.gov website (identifier NCT01899573), being conducted in Medellin, Colombia, last updated September 8, 2014, but there are no data reported.
Mitral cerclage annuloplasty
This approach is possibly the most creative among the methods for mitral annuloplasty. A wire loop is created encompassing the coronary sinus, basal myocardium and right atrial chamber (Figure 4)18. Annular tension is introduced through a “cerclage” suture that traverses the coronary sinus and basal septal myocardium and that is secured within the right atrium. Circumferential tension is intended to reduce annular dilation and enhance mitral leaflet coaptation by introducing radial force uniformly, irrespective of the rotational orientation of the commissures. For cerclage, 9 Fr introducer sheaths were placed percutaneously into the right jugular and femoral veins, and 6 Fr introducer sheaths into a femoral artery. A guidewire loop is created around the mitral annulus and LV outflow tract and then exchanged for a suture. The guidewire traverses the coronary sinus and the proximal great cardiac vein into the first septal perforator vein towards the basal interventricular septum. It is then directed across a short segment of myocardium to re-enter a right heart chamber where it is snared and exchanged for a suture and tension fixation device. To conduct cerclage, a transjugular balloon-tipped guiding catheter is introduced into the coronary sinus, the occlusion balloon inflated, and a retrograde coronary contrast venogram opacifies the great cardiac vein and septal perforator veins. A stiff 0.014” guidewire is steered into the first basal septal perforator vein. Once a right heart chamber is entered, the guidewire is snared and replaced with a braided non-absorbable tension suture.
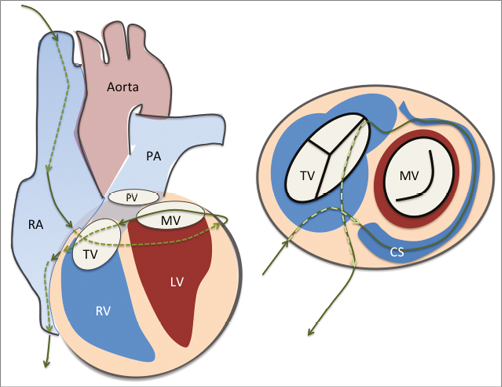
Figure 4. Cerclage annuloplasty. A guidewire is placed via the coronary sinus and then into a coronary vein. It is passed through the septal myocardium into the right ventricular outflow tract or right atrium to encircle the mitral annulus. CS: coronary sinus; LV: left ventricle; MV: mitral valve; PA: pulmonary artery; PV: pulmonic valve; RA: right atrium; RV: right ventricle; TV: tricuspid valve
A novel feature of the cerclage procedure incorporates the use of circumflex coronary artery protection. The coronary sinus frequently crosses over the circumflex or one of its branches19. The cerclage procedure utilises a relatively rigid spacer device, shaped somewhat like a piece of elbow macaroni. This spacer protects the coronary from compression by the cerclage loop.
Currently, a single-centre feasibility study is ongoing (ClinicalTrials.gov identifier NCT02471664) in Korea. The inclusion criteria include NYHA Class III-IV, and symptomatic severe functional MR despite optimal medical treatment. Optimal medical therapy includes an ACE inhibitor or angiotensin receptor blocker, β-blocker, and aldosterone antagonists for at least three months unless the subject has contraindication or intolerance for each drug. Some of the key exclusion criteria include LV ejection fraction lower than 25%, anomaly of the coronary sinus or pre-existing devices in coronary sinus such as implantable cardioverter defibrillator or pacemaker, or 2:1 or higher grade AV block.
Conclusions
Several direct annuloplasty devices have completed Phase II registry studies and are close to CE approval. It is reasonable to expect the beginning of larger, randomised pivotal trials, possibly for FDA approval over the next year. What will the role of these devices be compared to the MitraClip? How will they compare to transcatheter valve replacement20? With regard to the MitraClip, since the MitraClip can be used only when the leaflet anatomy is suitable, at the very least direct annuloplasty will have a role among patients with unsuitable anatomy for MitraClip. There is also the potential for combined use of leaflet and annular therapy, as primary or secondary therapy. The development of catheter-based valve replacement has raised the possibility that repair might become obsolete. The general principal that living with a prosthetic valve replacement is a second choice to effective repair is probably not changed by replacement devices, even if replacement devices are rapidly evolving and have few acute complications or long-term risks. In addition, the repair devices may have some direct effect on LV remodelling. Repair devices are thus likely to have an important role for the future. Another consideration for the individual patient is the impact of a repair implant on the future possibility for a transcatheter replacement. MitraClip creates an inflow obstruction by plicating the leaflets and may be “burning a bridge”, precluding future replacement, while placement of an annuloplasty ring may facilitate replacement by creating a landing zone for a valve prosthesis. The field of percutaneous mitral repair and replacement remains in the early stages, and the only certainty in the next few years is unanticipated consequences and outcomes.
Conflict of interest statement
T. Feldman receives institutional research grants and consulting fees from Abbott, Boston Scientific and Edwards. M. Guerrero receives institutional research grants and consulting fees from Edwards.

