Introduction
The Cardioband® (Valtech Cardio, Or Yehuda, Israel) is a transcatheter direct annuloplasty device that closely reproduces surgical annuloplasty. Following first-in-man experience, Cardioband use has been expanded and outcomes improved by a combination of improved patient selection and procedural refinements.
The implant
The Cardioband annuloplasty device is delivered via a transfemoral transvenous and transseptal route. It is implanted in the beating heart over the posterior side of the mitral valve annulus under fluoroscopic and echocardiographic guidance. The Cardioband is made of a polyester sleeve with radiopaque markers (ink stamped on the device) spaced 8 mm apart (Figure 1, Figure 2). The Cardioband implant is available in six lengths to cover a wide range of posterior annulus circumference sizes comparable to those of commercially available annuloplasty devices. During implantation, 12 to 17 metal anchors are inserted to fixate the Cardioband to the tissue. Each anchor is 6 mm long. The anchors are spiral-shaped with a screw-like fixating mechanism designed to secure the implant safely in place. All anchors are repositionable and retrievable until disconnected from the anchor drive. Once the Cardioband is successfully anchored along the posterior annulus, the band is dynamically cinched using a dedicated size-adjustment tool (SAT). The distal tip of the SAT is connected to an implant wire inside the Cardioband. The implant wire is foreshortened by gradually turning on the adjusting knob. Cinching of the band translates into a reduction of the mitral annular diameter of approximately 30%. The effect of reduced annular diameter on improved leaflet coaptation is guided by echocardiography.
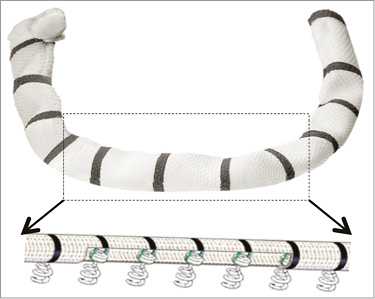
Figure 1. Cardioband implant. A Dacron band of variable length (according to patient annular dimensions in diastole) with radiopaque markers is attached with multiple anchors.
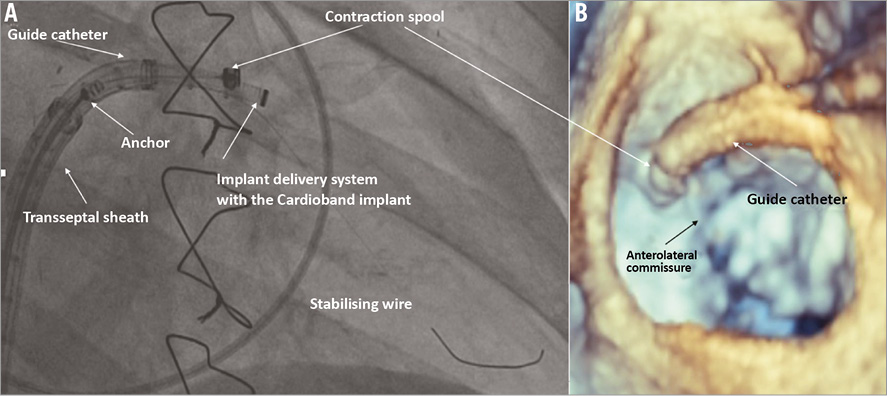
Figure 2. First anchor implant. The fluoroscopic anatomy of the device and of the delivery system (A), and the 3D echo appearance (B) at the time of the first anchor implant. Note the stabilising wire that supports the first anchor implantation.
Early clinical experience
The published data reported outcomes from 31 consecutively enrolled high-risk adult individuals at five institutions in Europe with symptomatic secondary mitral regurgitation (MR) despite optimal medical therapy and cardiac resynchronisation therapy (CRT) where indicated. Patients with a left ventricular (LV) ejection fraction ≤25% and an LV end-diastolic diameter ≥70 mm were excluded. Other exclusion criteria were organic mitral valve disease, calcification of the annulus, acute endocarditis, coronary artery disease requiring revascularisation, dialysis, significant concomitant valve disease, life expectancy <12 months, and recent cerebrovascular events. Both the serious adverse events (SAE) and the serious adverse device events (SADE) were independently adjudicated by a clinical events committee.
All the patients underwent pre-procedural TEE and cardiac computed tomography (CCT) to assess anatomical feasibility. CCT was instrumental for understanding annular anatomy, for measuring the mitral annulus, and for planning the procedure. In order to enable a safe and prompt procedure, CCT reconstructions revealed the ideal location of transseptal puncture and identified optimal fluoroscopic views for implanting the anchors. To mitigate the risk of injury to the left circumflex coronary artery, in-depth analyses of coronary anatomy and its relations to the annulus were performed in each patient. The implant size was decided on the basis of the length of the posterior annulus (from trigone to trigone) at mid diastole. The average implantation time was 2.45 hours and tended to become shorter with growing operator experience. Today, Cardioband implantation can be performed in roughly one hour in experienced hands.
All patients received the full implant of a Cardioband. Adjustment of the Cardioband resulted in a significant reduction in the septolateral dimension in all but two patients who experienced device failure. Following Cardioband contraction, the septolateral dimension was reduced from 37 mm (±4.8) to 29 mm (±5.5) (p<0.01). Excluding the two patients with device failure, MR was none or trace in six (21%), mild in 21 (72%), and moderate in two (7%). No patient had severe MR after adjustment. Procedural mortality was zero and in-hospital mortality was 6.5% (two of 31 patients). The two mortalities were not device-related. At 30 days, 88% of patients had MR ≤2+. These data have recently been confirmed in larger series. The reduction of MR was stable at 12 months, with 90% of patients having MR ≤2+ on TTE at one year (core lab adjudicated TTE images). The observed reduction of MR is associated with substantial and sustained improvement of quality of life and functional capacity.
Learning curve and improved outcomes
These data are surprising for a variety of reasons. The Cardioband experience is still early, and the learning curve has just started. However, the safety profile of this procedure replicates the high standards achieved by MitraClip therapy. The durability of mitral annuloplasty in functional MR is at least similar to (if not better than) that observed after surgical annuloplasty. Recent evidence has shown limited durability of undersized surgical annuloplasty, as compared to surgical valve replacement. Surprisingly, the rate of recurrence with Cardioband has been far superior to that shown in this trial. The difference may be determined by better selection, improved implantation technique, by the advantage of the echo-guided beating heart adjustment, or by the different anchoring system. Furthermore, implantation of an annuloplasty ring selectively along the posterior commissure may leave room for positive remodelling of the annulus after the procedure.
Patient selection has mainly focused on safety in the initial clinical experience. The main reasons for excluding patients from the trial were annular calcification and the vicinity of the circumflex artery to the annulus. With increased experience, most of these patients can be successfully treated today. To improve efficacy, the common predictors of success for surgical annuloplasty have also been considered valid in the case of the Cardioband (tenting angle, asymmetric tethering, etc.).
Quality of anchoring has been the first challenge in preclinical as well as in the very early phase of the clinical experience. Improved durability has been achieved with the introduction of longer anchors, increasing the density of anchors in the trigonal space, as well as by implementing a standardised anchor implantation quality control. The Cardioband is implanted with fixating anchors which penetrate into the base of the left ventricle. Differently from surgery in which the sutures are placed semi-blindly in the annular tissue, in the case of Cardioband each anchor is individually tested for positioning and quality of the implant, under echo and echo guidance. Each anchor is implanted as close as possible to the leaflet hinges, taking care to avoid leaflet or coronary lesions, and targeting the ventricular portion of the annulus. In most cases, during anchor implantation, it is possible to observe extrasystolic beats on the ECG as the anchors penetrate into the myocardial tissue. CT scan has been instrumental to improve knowledge about the anatomy of the annular structures in functional MR. Implantation of anchors with an angle of about 45 degrees has improved anchor fixation durability, and today anchor detachment is a very rare complication of the procedure.
Device iterations have been necessary to improve reliability of the complex system. Early in the experience, cinching was not effective in two patients due to device malfunction. This has been solved with engineering iterations and no more malfunctions have been observed in the last 50 implants.
An important contributor to the success of the procedure has been the implementation of a proper procedural standardisation including device manoeuvring and imaging guidance. This has allowed the procedure to be reproducible and doable without physician proctoring.
The potential to remodel the annulus opens the perspective of replicating surgical standards. As demonstrated by several series, the addition of annuloplasty to leaflet repair is associated with prolonged durability of the repair. The combination of leaflet repair and annulus remodelling is the gold standard in surgical repair of degenerative MR. In the future, it is not far from reality to predict that most patients undergoing leaflet repair in the context of degenerative MR will be treated with concomitant annuloplasty to improve early outcomes and prolong durability, in order to become competitive with surgical repair.
In the field of functional MR, transcatheter annuloplasty will compete against leaflet repair with the MitraClip® system (Abbott Vascular, Santa Clara, CA, USA), and against transcatheter replacement.
A comparison of Cardioband vs. MitraClip appears unfair today. MitraClip is the device with the largest and longest experience, while the Cardioband is just now entering the clinical arena. In functional MR, the Cardioband respects the mitral anatomy and physiology and it does not prevent further interventions, including MitraClip or mitral valve replacement.
Also, a direct comparison with percutaneous mitral valve replacement does not make sense, since most replacement technologies are in an early clinical or even preclinical stage, and there is room for improvement. However, early clinical experience of transcatheter replacement has been characterised by high perioperative mortality and morbidity, and the applicability of the therapy is currently limited by the availability of large devices and by the risk of left ventricular outflow tract obstruction. In addition, the durability of bioprostheses in the mitral position is questionable, and could limit its applicability as an early indication in heart failure patients.
Conclusion
The Cardioband is the first percutaneous transseptal annuloplasty device to achieve surgical standards. It is associated with consistent and durable reduction of the septolateral annular dimensions and of mitral regurgitation. Cardioband opens up the perspective for “surgical standard” transcatheter repair beyond palliation.
Conflict of interest statement
F. Maisano and A. Guidotti are consultants for Valtech Cardio. The other authorshave no conflicts of interest to declare.

