Introduction
Annuloplasty has been a mainstay of mitral valve surgery for decades. The implantation of rings, bands, or sutures has been used alone and in combination with other repair approaches in almost all mitral repair surgeries. Many different annuloplasty devices have been used and there are proponents and detractors for these varied approaches. Surgical annuloplasty has also coexisted with mitral valve replacement, with neither repair nor replacement supplanting the other. It is not surprising that percutaneous repair for mitral regurgitation (MR) has developed mostly by recapitulating surgical techniques. One difference for percutaneous annuloplasty has been the advent of both direct and indirect devices. Three of these, Carillon® (Cardiac Dimensions, Kirkland, WA, USA), Cardioband® (Valtech Cardio, Or Yehuda, Israel), and Mitralign (Mitralign, Inc., Tewksbury, MA, USA) are CE approved and are described elsewhere in this Supplement. This review will describe several additional, as yet unapproved, novel catheter-based annuloplasty devices, and also briefly describe some newer surgical annuloplasty concepts.
Direct annuloplasty
There are two CE approved percutaneous direct annuloplasty devices, the Cardioband (Valtech Cardio), and the Mitralign system (Mitralign, Inc.). The Cardioband most closely resembles a surgical annuloplasty, while the Mitralign system uses pledgets to plicate the mitral annulus, similar to some established surgical suture annuloplasty procedures1,2. Indirect annuloplasty has the potential to be simpler than direct annuloplasty, but direct approaches have the advantage of closer approximation of established surgical annuloplasty. Several other novel annuloplasty devices have been used preclinically, used with surgical implants, or are in early human use.
CERCLAGE ANNULOPLASTY
This creative method for mitral annuloplasty utilises a wire loop that is created by encompassing the coronary sinus, basal myocardium, and right atrial chamber (Figure 1)3. Annular tension is introduced through a “cerclage” suture that traverses the coronary sinus and basal septal myocardium and is secured within the right atrium. Circumferential tension is intended to introduce radial force uniformly to improve leaflet coaptation. For this procedure, 9 Fr introducer sheaths are placed in the right jugular and femoral veins, and 6 Fr introducer sheaths into a femoral artery. A guidewire loop is created around the mitral annulus and LV outflow tract and then exchanged for a suture. The guidewire traverses the coronary sinus and the proximal great cardiac vein into the first septal perforator vein towards the basal interventricular septum. It is then directed to perforate across a short segment of myocardium to re-enter a right heart chamber where it is snared and exchanged for a suture and tension fixation device. The coronary sinus frequently crosses over the circumflex or one of its branches. A unique feature of the cerclage procedure incorporates the use of circumflex coronary artery protection using a relatively rigid spacer device, shaped somewhat like a piece of elbow macaroni.
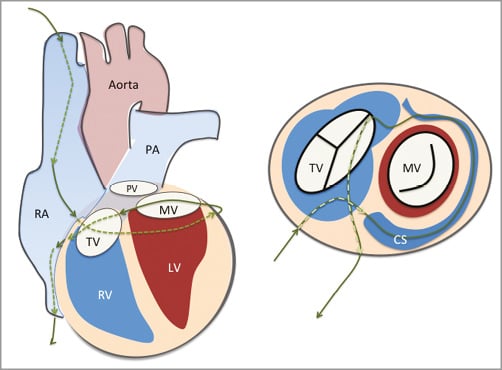
Figure 1. Cerclage annuloplasty. A guidewire is placed via the coronary sinus and then into a coronary vein. It is passed through the septal myocardium into the RV outflow tract or RA to encircle the mitral annulus. CS: coronary sinus; LV: left ventricle; MV: mitral valve; PA: pulmonary artery; PV: pulmonic valve; RA: right atrium; RV: right ventricle; TV: tricuspid valve From Feldman T, Guerrero M. Transcatheter direct mitral valve annuloplasty. EuroIntervention. 2015;11 Suppl W:W53-7.
A single-centre feasibility study is being conducted in Korea (ClinicalTrials.gov Identifier: NCT02471664). Inclusion criteria include NYHA Class III-IV symptoms with severe functional MR in conjunction with optimal medical therapy. Key exclusion criteria include LV ejection fraction lower than 25%, anomalies of the coronary sinus, or pre-existing coronary sinus devices such as a coronary sinus pacemaker lead or 2:1 or higher grade AV block. Several patients have been treated successfully.
MILLIPEDE
This is an annuloplasty ring that is anchored into the mitral (or tricuspid) annulus using screws, and is then mechanically cinched to reduce the mitral annular circumference (Figure 2). The Millipede system (Millipede, LLC, Ann Arbor, MI, USA) is a complete ring4. The cinching or adjustment is done in real time with echo guidance to optimise the reduction of MR. Several patients have had operative implants. A catheter system for percutaneous delivery is under development. An international trial, Annular Reshaping of the Mitral Valve for MR Using the Millipede IRIS System for symptomatic severe MR (effective regurgitant orifice area [EROA] ≥0.2 cm2 for secondary MR, EROA ≥0.4 cm2 for primary MR), is registered with ClinicalTrials.gov (Identifier: NCT02607527), anticipating enrolment of approximately ten patients, possibly beginning in 2016.
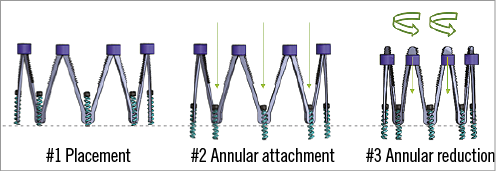
Figure 2. Millipede annuloplasty ring. A complete ring anchored into the mitral or tricuspid annulus using screws, and then mechanically cinched to reduce the annular circumference.
MITRASPAN TASRA
The Trans-Apical Segmental Reduction Annuloplasty (TASRA) technique (MitraSpan, Inc., Belmont, MA, USA) is based on the experimental concept of septal lateral annular cinching (SLAC). The SLAC concept was first described in adult sheep with acute ischaemic MR produced by circumflex occlusion. A suture is placed from the mid anterior annulus to the posterior LV wall (Figure 3). This resulted in an anteroposterior reduction of 22%5. Subsequent studies demonstrated MR elimination in a chronic ischaemic sheep model with preservation of both leaflet mobility and the normal saddle shape of the mitral annulus6,7. The feasibility of suture implants is generally established by the precedents of the components of the procedure, including neochordal implants, the Myocor device8, and suture annuloplasty.
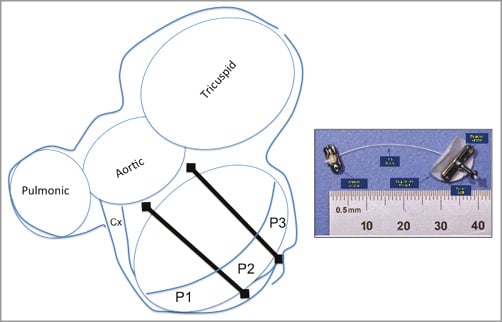
Figure 3. Trans-Apical Segmental Reduction Annuloplasty (TASRA). A suture is placed from the mid anterior annulus to the posterior LV wall to accomplish annular cinching.
The device system is transapical and relatively low-profile (maximum 12 Fr). It allows a direct approach to the critical locations for device implantation using short tools. Tissue crossing uses a 0.018” wire. There are pledgeted anchors on the LV side and by the trigones. The procedure is TEE guided, with pre-procedure planning with multislice CT.
The safety of implants and biocompatibility of the polyester suture and stainless steel anchors have been demonstrated in preclinical studies of 60 acute and chronic porcine implants with up to 12 weeks pathology and in 18 consecutive chronic animal implants up to 30 days. In these preclinical studies, efficacy to reduce the annular diameter by 20-40% was confirmed.
A human feasibility study of the MitraSpan device (SPARE-MR) has been initiated outside the USA. The inclusion criteria include moderate-severe, symptomatic, secondary MR with LVEF 20-50%. An IDE early feasibility study has been approved by the FDA.
GUIDED DELIVERY SYSTEMS
The Accucinch® System (Guided Delivery Systems, Santa Clara, CA, USA) implants multiple anchors in the mitral basal annular myocardium. A tether connects the anchors, and tension of the catheter diminishes the mitral annular circumference (Figure 4). This approach results in both diminution of the annular circumference, and at the same time remodelling of the basal LV myocardium. The Accucinch device places a delivery system below the posterior mitral leaflet on the ventricular side, with delivery of 10 and 20 nitinol anchors. These anchors are tethered by a drawstring to reduce the mitral annulus circumference. Several patients have been treated over the last few years as the system has evolved, but there is no published experience.
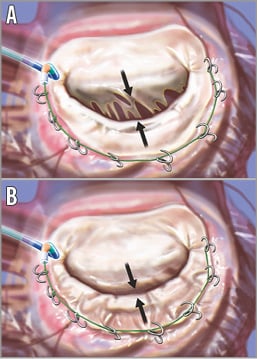
Figure 4. The Accucinch device. The device is delivered through retrograde catheterisation of the LV (A). The arrows highlight the separation of the leaflet edges, which define the regurgitant orifice area. Anchors are placed in the posterior mitral annulus and connected with a “drawstring” to cinch the annulus. When the cord is tightened, the basilar myocardium and annulus draw the mitral leaflets together to decrease the regurgitant orifice area (B). Artwork by Craig Skaggs. From Feldman T, Young A. Percutaneous approaches to valve repair for MR. J Am Coll Cardiol. 2014;63:2057-68.
QUANTUMCOR
The QuantumCor (QuantumCor, Inc., Lake Forest, CA, USA) system uses a radiofrequency (RF) system to shrink collagen, resulting in remodelling of the annulus. The RF energy is administered with electrodes that are delivered transseptally. In preclinical work described in 2008, the device was evaluated in sixteen animals9. Acutely, all responded appropriately with an average septal-lateral reduction of 21.7%. Seven animals survived for four to 180 days where the average septal-lateral reduction was 26.5%, with some expectation that additional remodelling might occur as the collagen matrix healed.
VALCARE
Valcare Medical (Herzliya Pituach, Israel) has developed a minimally invasive ring technology, the Amend™ device, which emulates a surgical closed, semi-rigid D-shaped annuloplasty ring (Figure 5)10. The system is delivered via apical LV access into the mitral annulus. The implant is delivered under fluoroscopic and echo guidance in a linear configuration through a catheter to the target site. As it is advanced from the guide catheter system, it changes geometry above the annulus using a series of remotely activated mechanisms. The result is a complete D-shaped ring. A series of 12 anchors in four zones that are independently deployed attach the ring into the annulus. The posterior anchors are placed first, and then pulled towards the anterior side to reduce the anteroposterior dimension, and thus reduce the septal-lateral dimension and mitral circumference. The technology creates an opportunity to be used as a platform for mitral valve replacement, possibly using a valve prosthesis proven in the aortic position. Several patients have been treated on a compassionate use basis.
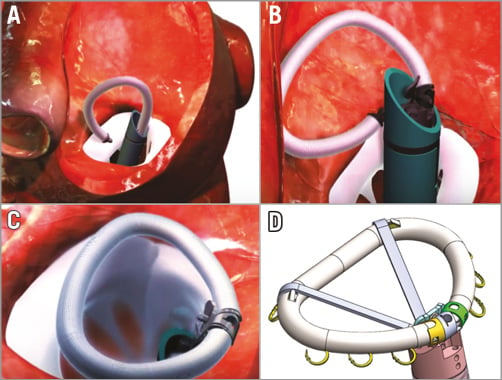
Figure 5. The Amend device emulates a closed surgical semi-rigid D-shaped annuloplasty ring. A) The system is delivered via apical LV access into the mitral annulus in a linear configuration through a catheter to the target site. B) As it is advanced it changes geometry above the annulus using a series of remotely activated mechanisms (https://www.youtube.com/watch?v=tQY-BJZpKKw). C) The result is a complete D-shaped ring. D) A series of 12 anchors in four zones that are independently deployed attach the ring into the annulus.
Indirect annuloplasty
The Carillon coronary sinus (CS) implant (Cardiac Dimensions) is the only indirect annuloplasty device with CE approval and undergoing clinical use. The CS is attractive for annuloplasty because it encircles a significant portion of the annular circumference, and also is easily accessible using jugular venous puncture. This apparent simplicity is complicated by complex torsional deformation of the CS, which resulted in two indirect annuloplasty systems previously failing, due to device fracture or coronary sinus erosion. The MONARC device (Edwards Lifesciences, Irvine, CA, USA) was a spring-like band anchored in the CS with self-expanding stents at either end. The connection between the band and the stents fractured in several cases. The Viacor PTMA® system (Viacor, Inc., Wilmington, MA, USA) used nitinol rods to compress the posterior mitral annulus from within the CS. In one patient treated with this device a fracture resulted in CS laceration11.
ARTO™ SYSTEM
The Arto System (MVRx, Inc., Belmont, CA, USA) is comprised of two anchors, deployed in the lateral wall of the left atrium via the CS and in the atrial septum and connected by a tether that traverses the left atrial chamber12.
This was previously known as the PS3 System. The tether between the two anchors provides the means for reduction of the minor axis of the mitral valve (Figure 6). The procedure is performed under general anaesthesia with fluoroscopic and transoesophageal echocardiographic guidance. Using magnetically linked catheters and routine catheter exchanges, an anchor (T-Bar) is placed in the CS and connected by the tether to an atrial septal anchor. The tether is tensioned to decrease indirectly the anteroposterior diameter of the mitral annulus, which results in reduction of MR. Since the implant is atrial there is no haemodynamic instability and there are no ventricular arrhythmias. Device efficacy is seen immediately at the time of implantation. The system is recapturable and retrievable during the deployment.
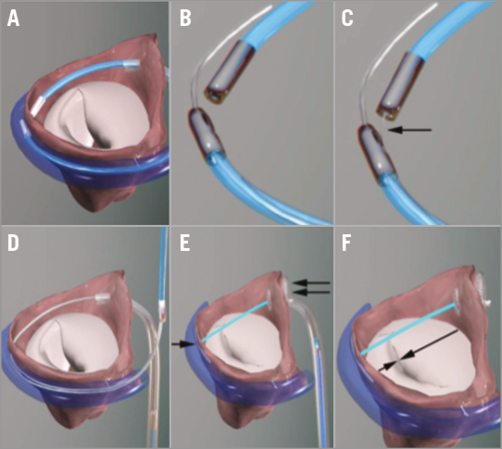
Figure 6. The Arto System implantation procedure. A) Great cardiac vein (GCV) and left atrial (LA) MagneCaths (MagneCath™; Ample Medical, Foster City, CA, USA) in position and magnetically linked behind the P2 segment of the posterior mitral leaflet. B) Magnetically linked LA and GCV MagneCaths. C) The crossing wire (arrow) is pushed from the GCV into the LA MagneCath and aligned to direct the wire to the LA through the atrial wall. D) After using an exchange catheter, the loop guidewire is in place across the left atrium to direct the placement of the GCV and septal anchors. E) The Arto System in place before tensioning. T-Bar, single arrow; septal anchor, double arrow. F) Tensioning of the bridge results in shortening of the mitral annulus anteroposterior diameter (arrows). Once the final position is attained, the suture is cut and secured with a suture lock.
Clinical data from eleven patients have been published: the MitrAl ValvE Repair Clinical Trial (MAVERIC Trial; ClinicalTrials.gov Identifier: NCT02302872)13. The patients were all high-risk as judged by a Heart Team and were symptomatic. There were no procedural safety events, and two clinical events within 30 days of follow-up. One patient underwent surgical drainage of pericardial effusion without recurrence and one patient had asymptomatic dislocation of the coronary sinus T-Bar, finally treated with elective surgical MV replacement. At six months, MR grade, LV volumes, mitral annular dimensions and functional status were all improved. Pre-procedure FMR was grade 3-4+ in 90%, and after six months decreased to grade 1-2+ in 80%. EROA by PISA at baseline was 30.3±11.1 mm², decreasing at six months to 13.7±8.6 mm2. Regurgitant volumes decreased from 45.4±15.0 to 19.9±11.6 mL. LVESVi decreased from 77.5±24.3 to 68.2±28.2 mL/m2 and LVEDVi from 118.7±28.6 to 104.9±30.2 mL/m2 at six months. Mitral annular anteroposterior diameter decreased from 45.0±3.3 to 38.9±2.7 mm. Functional status was NYHA Class III or IV in 81.8% at baseline, improving at six months to 50% Class III and 50% Class I/II. Enrolment of up to 20 additional patients at three sites (Riga, Latvia, Massy, France and London, United Kingdom) is underway in Phase II of the MAVERIC Trial.
MARDIL EXTRACARDIAC ANNULOPLASTY
Basal Annuloplasty of the Cardia Externally (BACE™; Mardil Medical, Inc., Plymouth, MN, USA) is a tension band with inflatable silicone chambers that is wrapped around a section of the heart and as an extracardiac “jacket” (Figure 7). BACE is an extracardiac device and does not require open heart surgery for placement. A tool to implant the BACE device through a small incision is under development as a minimally invasive procedure. After the device is sutured to the heart, the silicone chambers are filled with saline via tubing connected to subcutaneous ports. The saline applies pressure at the basal LV to approximate the mitral leaflets. The saline levels can be adjusted during or after the procedure to provide the appropriate pressure needed to minimise functional MR. The pilot clinical study enrolled 11 patients at medical centres in India and showed clinical efficacy and no device-related safety concerns. Results in five patients have been published14. All five subjects were male, in NYHA Class III, with LVEF of 20-40%. Epicardial application and adjustment of the BACE device was performed during coronary bypass surgery on the beating heart with effective reduction in functional MR to grade <1. All five subjects also had coronary bypass grafts. Reduction in MR was sustained for at least six months and there were no unanticipated or device-related adverse events. The system is being further studied in a 14-patient trial, the Evaluation of the Minimally Invasive VenTouch System in the Treatment of Functional MR (ClinicalTrials.gov Identifier: NCT02671799) with centres recruiting in France and Malaysia, and additional centres planned in Canada, Czech Republic and The Netherlands. There is also progress in a CE-mark study with 30 implants performed worldwide out of a total intended enrolment of 50 patients.
Figure 7. BACE. BACE is a tension band with inflatable silicone chambers that is wrapped around a section of the heart and is thus an extracardiac device. After the device is sutured to the heart, the chambers are filled with saline via tubing connected to subcutaneous ports.
Novel surgical techniques with implications for catheter approaches
Several new surgical techniques that introduce novel repair concepts and/or have implications for less invasive device development have been reported.
enCOR™ ADJUSTABLE SURGICAL ANNULOPLASTY RING
This is currently a surgical annuloplasty ring with a percutaneous adjustment mechanism (MiCardia Corp., Irvine, CA, USA)15. The concept is that late postoperative adjustment of the ring could be an advantage. The ring is deformable, nickel-titanium based. It is heated up for 45 seconds, which induces a change of geometry to a preformed reduced anterior-posterior diameter.
In a clinical trial of 94 patients, a smaller ring size was implanted in patients with ischaemic MR to “downsize” the mitral annulus16. A permanent lead was attached to the ring in the P2/P3 region. It was routed through the atrial wall to a subcutaneous pocket. If an adjustment was required, the lead was accessed by a small incision, and connected to a generator. The ring adjusted its form during the activation procedure to a preformed shape with a reduced anterior-posterior diameter. The median age was 71 (range 64-75) years with a EuroSCORE II of 6.7±6.3. Two thirds were male, 48% had ischaemic MR, 37% had dilated cardiomyopathy and 15% degenerative disease. Operative mortality was 1% and the one-year survival was 93%. Ring adjustment was attempted in 12 patients at a mean interval of 9±6 months after surgery. In three of these attempts, a technical failure occurred. In one patient, MR was reduced by two grades, in two patients MR was reduced by one grade and in six patients MR did not change significantly. The mean grade of MR changed from 2.9±0.9 to 2.1±0.7 (p=0.02). Five patients were re-operated after 11±9 months (ring dehiscence: 2; failed adjustment: 3). Based on this early experience, the authors concluded that an adjustable ring may provide an additional option for recurrent MR. A percutaneous version, enCorTC, is under development.
MITRAL BRIDGE™
The Mitral Bridge (Heart Repair Technologies Inc., Menlo Park, CA, USA) is a novel surgical annular repair device. Rather than encircle the mitral annulus with a ring, a nitinol bridge is sutured from the septal to the lateral annulus (Figure 8). It is placed between A2 and P2 at annular level with standard sutures. It is available in five sizes from 22-30 mm. Thirty-four patients have been treated in a CE approval trial. Three quarters had Type I MR with annular dilatation, and one quarter had Type III-b ischaemic MR. Chronic AF was present in 88%. All 34 successful implants experienced a decrease of MR to <1+. One patient developed a paravalvular leak and required reoperation at seven months post implantation, and one developed a paravalvular leak and required catheter closure after 18 months. The device is unique in that it reduces and stabilises the anteroposterior annular dimension without the use of annular trigone-to-trigone anchoring. It has been hypothesised that part of the mechanism of action for MitraClip® (Abbott Vascular, Santa Clara, CA, USA) is anteroposterior annular stabilisation by the tissue bridge that develops after edge-to-edge repair17.
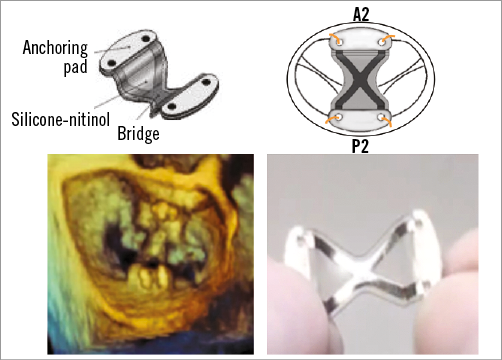
Figure 8. Mitral Bridge. Mitral Bridge is a novel surgical annular repair device that is sutured from the septal to the lateral annulus. It is placed between A2 and P2 at annular level with standard sutures.
Conflict of interest statement
T. Feldman receives research grants and honoraria from Abbott, BSC, Edwards and WL Gore. M. Guerrero receives research grants and honoraria from Edwards. M. Salinger receives honoraria from BSC.

