Abstract
Over the last decade, percutaneous intervention for the treatment of aortic stenosis has become commonplace with >300,000 implantations performed worldwide. With this now being an established therapy, focus has shifted to the more intricate challenge of mitral and tricuspid valve disease, where there remains a large population of patients with unmet clinical needs. These complex anatomical structures demand unique approaches to treat a wide range of pathologies involving the valve leaflets, annulus and chordae. A large armamentarium of devices is under evaluation in preclinical animal studies or preliminary clinical trials. Herein, we review the technical characteristics of mitral and tricuspid devices in current clinical application and summarise the available data concerning their use in humans.
Introduction
Mitral regurgitation (MR) is prevalent in 1.7% of the general population and 10% of individuals over 75 years of age1. MR can arise from abnormalities of the valve itself (primary or degenerative MR) but is more commonly a consequence of underlying left ventricular (LV) dysfunction, which results in restricted leaflet motion and inadequate leaflet coaptation (secondary or functional MR). Although open heart surgery is currently the gold standard treatment, 50% of patients with severe MR are not offered surgery in view of severe LV dysfunction or other comorbidities2.
Tricuspid regurgitation (TR) is usually associated with left-sided valve disease or pulmonary hypertension and is poorly recognised and frequently overlooked, despite being associated with symptoms of right heart failure, reduced quality of life and poor outcome3. Surgical repair is rarely performed as a stand-alone procedure since the impact on clinical outcomes is unclear.
Following the rapid expansion of transcatheter aortic valve implantation (TAVI) there has been active development in percutaneous mitral and tricuspid technologies to address this unmet clinical need. In this article, we summarise those that have been investigated in humans.
Mitral valve devices (Figure 1)
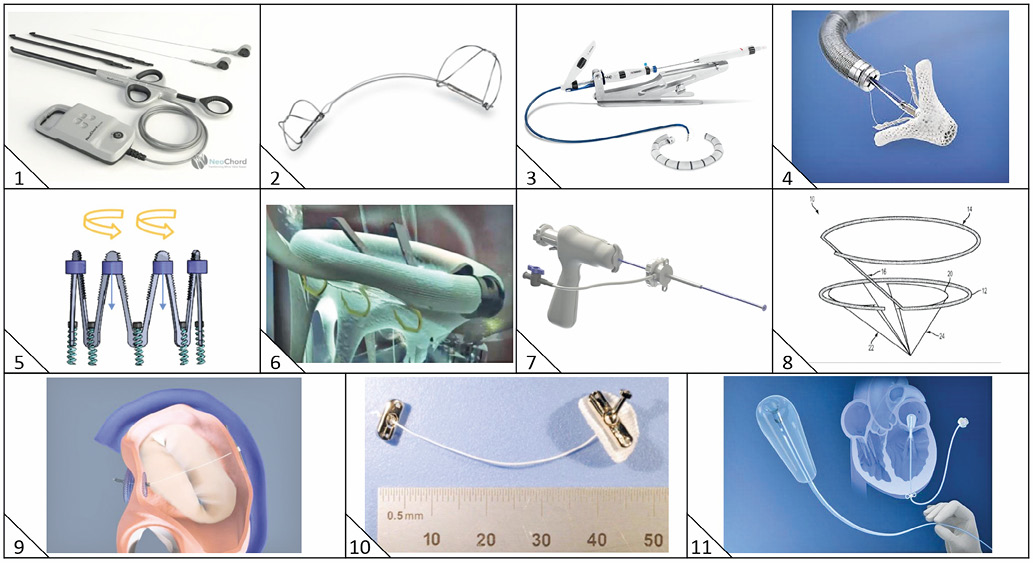
Figure 1. Transcatheter mitral valve repair devices. 1) NeoChord DS1000 (NeoChord Inc., St. Louis Park, MN, USA). 2) Carillon Mitral Contour System (Cardiac Dimensions Inc., Kirkland, WA, USA). 3) Cardioband (Edwards Lifesciences, Irvine, CA, USA). 4) MitraClip (Abbott Vascular, Santa Clara, CA, USA). 5) IRIS (Millipede Medical, Santa Rosa, CA, USA). 6) Amend (Valcare Medical, Herzliya Pituach, Israel). 7) TSD-5 (Harpoon Medical, Baltimore, MD, USA). 8) MISTRAL (Mitralix Ltd., Rehovot, Israel). 9) ARTO (MVRx, Inc., Belmont, CA, USA). 10) TASRA (MitraSpan Inc., Belmont, MA, USA). 11) Mitra-Spacer (Cardiosolutions Inc., West Bridgewater, MA, USA).
The principles of transcatheter mitral valve repair are based on those of surgery, including neochordae placement, leaflet plication, and annuloplasty4. However, reduction of MR is less predictable using percutaneous techniques and MR may persist or recur. Furthermore, these procedures require mastery of multiple transcatheter techniques5.
Although transcatheter mitral valve replacement is potentially applicable to a greater proportion of patients, the complex anatomy and pathophysiology of the mitral valve4,6,7 provide additional challenges when compared to routine TAVI procedures.
The MitraClip® (Abbott Vascular, Santa Clara, CA, USA), which mimics the Alfieri edge-to-edge leaflet repair, is the most widely used transcatheter mitral valve repair device and has been used to treat >35,000 patients. Landmark randomised trials evaluating its safety and efficacy in comparison with optimal medical therapy for patients with functional mitral regurgitation are approaching completion and their results will determine further progress in the field.
Chordal repair
NEOCHORD DS1000
The NeoChord DS1000 (NeoChord Inc., St. Louis Park, MN, USA) device enables off-pump, transapical implantation of neochordae by attaching expanded polytetrafluoroethylene (ePTFE) sutures to the prolapsing mitral valve leaflet under echocardiographic guidance8. The leaflet is grasped, then pierced to allow fixation and retraction of the neochordae, before fixation at the LV apex. The Transapical Artificial Chordae Tendinae (TACT) trial enrolled 30 patients with severe MR due to isolated posterior prolapse. Procedural success (defined as placement of at least one neochord and reduction of MR from 3-4+ to ≤2+) was achieved in 26 patients (86.7%), and 17 patients (65.4%) maintained MR grade ≤2+ at 30 days8.
HARPOON TSD-5
The Harpoon TSD-5 (Harpoon Medical, Baltimore, MD, USA) is a 3 mm diameter shafted instrument designed to anchor ePTFE cords onto the prolapsed mitral leaflet9 via transapical access and a 14 Fr valved introducer. The Harpoon TSD-5 is directed to the ventricular surface of the prolapsed leaflet under transoesophageal echocardiography (TOE) guidance, stabilised and then actuated, resulting in perforation of the leaflet by a needle wrapped with 50 coils of ePTFE in a pre-formed knot configuration. As the needle is withdrawn, a double-helix coiled ePTFE knot is formed on the atrial surface of the leaflet to secure the associated pair of ePTFE artificial cords to the leaflet. Additional Harpoon TSD-5 devices (typically three or four) may be deployed as required before final length selection (to ensure maximal coaptation) and tethering at the LV apex.
To date, outcomes of 11 patients with posterior leaflet prolapse and severe MR have been reported with 100% procedural success and minimal post-procedural MR. At one month, mean MR grade was mild with significant reduction in end-diastolic (139 to 107 ml, p=0.03) and left atrial volume (118 to 85 ml, p=0.04)9.
MISTRAL®
The transseptal 12 [Fr] MISTRAL device (Mitralix Ltd., Rehovot, Israel) is an atraumatic spiral-shaped nitinol wire that pulls the chordae together5,10.
Direct annuloplasty
CARDIOBAND
The Cardioband (Edwards Lifesciences, Irvine, CA, USA) adjustable annuloplasty system11 consists of a polyester sleeve implant (with radiopaque markers spaced 8 mm apart and available in six lengths), a 25 Fr transseptal steerable sheath, a transfemoral implant delivery system with the Cardioband implant mounted at its distal end, repositionable and retrievable 6 mm anchors which are used to fasten the Cardioband implant to the mitral valve annulus, and a size adjustment tool.
The procedure is performed via transseptal puncture under TOE guidance. The first anchor is released close to the leaflet hinge and anterior commissure and the Cardioband implant deployed until the radiopaque portion reaches the adjacent marker. The implant catheter is then navigated to the next anchoring point along the posterior annulus and these actions repeated until the implant catheter tip reaches the last anchoring site on the posterior commissure. Following deployment of the last anchor, the implant is tightened using the size adjustment tool and concomitant reduction of MR assessed by TOE.
Feasibility and safety have been demonstrated recently11 in 31 high-risk patients with symptomatic functional MR. Procedural success was obtained in 100% with significant reduction in septolateral dimension in 29 patients (36.8±4.8 to 29±5.5 mm, p<0.01). Following Cardioband adjustment (29/31), MR was absent in six (21%), mild in 21 (72%), and moderate in two (7%). There was no procedural mortality and in-hospital mortality was 6.5% (2/31, neither procedure- nor device-related). At 30 days, MR remained ≤2+ in 22/25 patients (88%).
AMEND™
Amend (Valcare Medical, Herzliya Pituach, Israel) is a D-shaped annuloplasty ring delivered via transapical approach12 and attached to the annulus using 12 independently deployed anchors positioned in four zones. The posterior zones are anchored first and pulled anteriorly to reduce the anteroposterior dimension by 15-25%. The first human implantation was announced in 2016. Available at: http://www.valcaremedical.com/valcare-touts-1st-in-human-use-of-amend-annulplasty-ring. This reduced MR in the patient from severe (4+) to trace (1).
TRANSAPICAL SEGMENTED REDUCTION ANNULOPLASTY (TASRA)
TASRA (MitraSpan Inc., Belmont, MA, USA) is a transapical, low-profile (maximum 12 Fr) annuloplasty device12 (Herrmann H. MitraSpan. Transapical-Segmented Reduction Annuloplasty [TASRA]. Presented at Transcatheter Valve Therapies 2016, Chicago, IL, USA, June 2016. Available at: https://www.tctmd.com/slide/mitraspan-suture-based-annuloplasty) based on the concept of septal lateral annular cinching (SLAC) and entailing placement of two sutures which span and cinch the mitral annulus from the anterior trigones to the posterior annulus.
IRIS
The IRIS (Millipede Medical, Santa Rosa, CA, USA) is a semi-rigid complete ring12 with a zigzag nitinol frame which is placed in a supra-annular position via transseptal approach with anchors to attach the ring to the annulus. Each zigzag peak has individually controlled collars that contract or expand the ring when tightened or released, respectively, thereby determining final sizing. Successful first-in-human implantation was announced in May 2017. Available at: http://www.prnewswire. com/news-releases/millipede-inc-announces-successful-clinicaluse-of-the-first-adjustable-retrievable-transcatheter-completemitral-annuloplasty-ring-300456710.html?tc=eml_cleartime
Indirect annuloplasty
CARILLON® MITRAL CONTOUR SYSTEM®
The Carillon system (Cardiac Dimensions Inc., Kirkland, WA, USA) is a fixed length, double-anchor, nitinol device that is positioned in the coronary sinus to reduce functional MR13. The distal anchor is deployed deep in the coronary sinus via a 9 Fr delivery catheter. Traction results in cinching of the posterior periannular tissue and reduction of the mitral annular circumference. Once optimal tissue plication is confirmed, the proximal anchor is deployed at the ostium of the coronary sinus, followed by angiography to confirm patency of the circumflex coronary artery before the device is finally released.
In the CARILLON Mitral Annuloplasty Device European Union Study (AMADEUS)14, two thirds (n=30/48) of enrolled patients received the Carillon device with only modest reduction in MR (22%) at six months (assessed using five different echocardiographic measures). Similarly, in the Transcatheter Implantation of Carillon Mitral Annuloplasty Device (TITAN) trial14, device implantation was successful in 68% (n=36/53) of patients and associated with significant reduction in regurgitant (34.5±11.5 ml vs. 17.4±12.4 ml, p<0.001), LV diastolic (208.5±62.0 ml vs. 178.9±48.0 ml, p<0.02) and systolic volumes (151.8±57.1 ml vs. 120.7±43.2 ml, p<0.02), and improvement in six-minute walk distance (102.5±64 m and 131.9±80 m at 12 and 24 months, respectively [both p<0.02]). Despite modest results, the Carillon’s merit lies in the relative simplicity of its design.
ARTO™
The ARTO (MVRx, Inc., Belmont, CA, USA) device is designed to treat functional MR and consists of a suture connecting interatrial-septal and coronary sinus anchors. When tensioned, this suture shortens the anteroposterior diameter of the mitral annulus, improves leaflet coaptation and reduces MR15.
Magnet-tipped catheters positioned in the left atrium (via transseptal puncture) and coronary sinus link on either side of the atrial wall behind the P2 segment of the posterior leaflet and are joined by a crossing wire to facilitate placement of a T-bar anchor in the coronary sinus. An atrial-septal anchor is then deployed at the site of transseptal puncture made during the procedure and the linking suture tensioned under TOE guidance to achieve precise shortening of the mitral annular anteroposterior diameter and reduction of MR.
In the first phase of the MitrAl ValvE RepaIr Clinical Trial (MAVERIC, ClinicalTrials.gov identifier: NCT02302872), 11 consecutive NYHA Class II-III patients with MR grade ≥2+ underwent successful ARTO system implantation. All were deemed prohibitively high surgical risk by the Heart Team and highly symptomatic (82% NYHA Class III/IV). There were no procedural safety events and only two clinical events at 30-day follow-up (one pericardial effusion requiring surgical drainage, one asymptomatic device dislodgement)16. At six-month follow-up, there were improvements in MR grade (baseline 90% grade 3-4+, 6 months 80% grade 1-2+), LV volumes, MR severity (PISA baseline 30.3±11.1 mm2, six months 13.7±8.6 mm2), regurgitant volumes, annular diameter, and functional status (baseline NYHA Class III/IV 82%, Class I/II 18%, six months class III 50.0%, class I/II 50.0%). Phase II trial enrolment is underway in multiple sites worldwide.
Leaflet coaptation enhancement
MITRA-SPACER™
The transapical (18 Fr) Mitra-Spacer system (Cardiosolutions Inc., West Bridgewater, MA, USA) comprises a volume-adjustable fluid-filled balloon attached to an apical anchor. The balloon acts as a space-occupying occluder to reduce regurgitant orifice and allows future size adjustments via a subcutaneous port.
Preliminary experience in a compassionate case has demonstrated proof of concept with survival to allow subsequent cardiac surgery17. However, thrombus formation despite oral coagulation has led to temporary suspension of the clinical programme.
Mitral valve replacement (Figure 2)
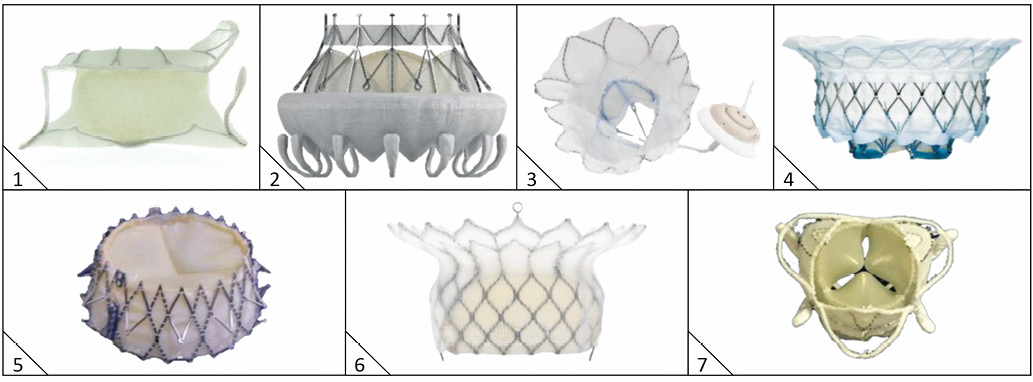
Figure 2. Transcatheter mitral valve replacement devices. 1) Tiara (Neovasc Inc., Richmond, BC, Canada). 2) CardiAQ (Edwards Lifesciences, Irvine, CA, USA). 3) Tendyne (Abbott Vascular, Santa Clara, CA, USA). 4) Intrepid (Medtronic, Minneapolis, MN, USA). 5) Navi (NaviGate Cardiac Structures Inc., Lake Forest, CA, USA). 6) HighLife (HighLife SAS, Paris, France). 7) Caisson TMVR (Caisson Interventional LLC [now LivaNova], Maple Grove, MN, USA).
`
INTREPID™
The Intrepid system (Medtronic, Minneapolis, MN, USA) comprises a self-expanding, nitinol frame, trileaflet bovine pericardial valve and a transapical hydraulic delivery system which assists with controlled expansion and deployment18. The prosthesis has a unique dual structure, consisting of an inner circular stent to house the valve and a conformable outer fixation ring. The outer ring engages with the dynamic mitral annulus whilst isolating the inner valve assembly throughout the cardiac cycle. The bioprosthesis has a 27 mm inner valve structure (effective orifice area 2.4 cm²) and is being investigated in 43 mm, 46 mm, and 50 mm outer diameters. Fixation and sealing is achieved through a combination of design features: 1) the outer fixation ring is larger in circumference than the native MV annulus with varying radial stiffness, 2) the atrial portion of the outer ring is flexible (allowing conformation to the native annulus) whereas the stiffer ventricular portion resists compression, producing a final “champagne cork” conformation to resist migration under systolic pressure, 3) the outer and inner stent frames are covered by a polyester fabric skirt to prevent paraprosthetic leaks and facilitate tissue ingrowth. The prosthesis has minimal protrusion below the annulus to avoid LV outflow tract obstruction. Furthermore, the system does not require rotational alignment, tethering, or capture of native leaflets before or during device deployment. Over 40 patients have now been treated in an ongoing international multicentre clinical study.
CARDIAQ
The CardiAQ system (Edwards Lifesciences, Irvine, CA, USA) consists of a self-expanding, nitinol frame, trileaflet bovine pericardial valve and 33 Fr delivery system for transapical or transseptal use19. The bioprosthesis frame has opposing anchors that secure the device in the mitral annulus, foam-covered ventricular anchors that engage and preserve the subvalve apparatus and a polyester fabric skirt to minimise paraprosthetic regurgitation. Supra-annular positioning reduces the risk of LVOT obstruction and there is no need for rotational alignment.
First-in-human implantation was performed in 201220 and first transfemoral implantation of the second-generation device in February 201621. From May 2014 to June 2016, approximately 15 second-generation devices have been implanted via transapical and transseptal approaches22.
TENDYNE
The Tendyne system (Abbott Vascular, Santa Clara, CA, USA) comprises a transapical, fully repositionable and recapturable trileaflet porcine pericardial valve sewn within two self-expanding nitinol stents, and an LV apical tethering system23. The outer stent, available in different sizes, is D-shaped to conform to the native mitral annulus and the inner circular frame is one size, maintaining a large effective orifice area (>3.0 cm2). The LV apical tethering system is designed to reduce paravalvular regurgitation and assist apical closure.
A 34 Fr apical delivery sheath is advanced to the mid-orifice of the mitral valve (clear of the subvalve apparatus) followed by positioning of the valve above the mitral annulus. The D-shaped Tendyne outer stent is oriented such that the straight side and atrial cuff are aligned with the aortomitral continuity and aorta. The valve is retracted into the mitral annulus (with tactile feedback) and positioning confirmed with TOE. Finally, the epicardial apical pad is positioned over the tether at the LV apex and a tension gauge used to adjust tension and the length between the valve and the apex (under TOE guidance) to ensure valve stability.
In a global feasibility study of 30 patients, device implantation was successful in 28/30 patients (93.3%) with grade 3/4 MR24. One patient died 13 days after implantation from hospital-acquired pneumonia with no residual MR in 26 patients and only mild central MR (1+) in one patient at 30 days. At follow-up, 75% of patients were in NYHA Class I-II with significant reduction in LV end-diastolic volume index (72.1±19.3 ml/m2 vs. 90.1±28.2 ml/m2, p=0.0012).
HIGHLIFE™
The HighLife system (HighLife SAS, Paris, France) consists of a nitinol frame, trileaflet, bovine pericardial valve which has a pre-formed annular groove to allow seating within a subannular ring-like implant25. An initial 18 Fr femoral arterial sheath enables retrograde advancement of a loop via the aortic valve and delivery of the subannular implant. Secondary access (transatrial or transapical) allows insertion of the mitral valve delivery catheter and advancement through the native valve leaftlets and subannular implant. The subannular implant (which is loose around the bioprosthesis to prevent movement into the atrium) and the shape of the bioprosthesis (which prevents movement into the ventricle) ensure stability. In a single-centre early feasibility clinical trial, the first patient was treated successfully with no paravalvular regurgitation, a mean transvalvular gradient of 3 mmHg and effective orifice area of 3.3 cm226. The results of six implantations were presented at TCT 2016 – five successful implantations (one death on day 4 with multi-organ failure) and one conversion to surgical MVR (death on day 4 due to multi-organ failure). Outcomes at 30 days in the remaining four patients were satisfactory (all ≤mild MR, ≤NYHA Class II) (Lange R. A two-component, self-centering TMV system. Presented at: Transcatheter Cardiovascular Therapeutics [TCT], Washington, DC, USA, 31 October 2016. Available at: https://www.tctmd.com/slide/highlife-design-and-clinical-trialupdates).
TIARA™
The Tiara (Neovasc Inc., Richmond, BC, Canada) is a D-shaped, self-expanding, nitinol frame, trileaflet bovine pericardial valve with a full atrial skirt and three ventricular anchors (one anterior, two posterior) that fix the valve onto the fibrous trigone and posterior annulus27. The valve is delivered via transapical approach (32 Fr for the 35 mm valve, 36 Fr for the 40 mm valve)28, and resheathing, repositioning and retrieval are possible until the final stage of deployment. The first-in-human implantation was performed in January 201429 and TIARA-I (30-patient early feasibility study) and TIARA-II (115-patient prospective CE mark trial) are ongoing.
NAVI™
The Navi Mitral valve (NaviGate Cardiac Structures Inc., Lake Forest, CA, USA) is a self-expanding nitinol frame valve (Navia J. My choice for percutaneous mitral valve replacement. Presented at: American Association for Thoracic Surgery Annual Meeting, Baltimore, MD, USA, 25 April 2015. Available at: http://webcast.aats.org/2015/Presentations_2/6B/04252015/1300-Adult%20Cardiac%20S2/1356_Navia_J/AATS_PMVR.pdf), available in three sizes (30/36 mm, 30/40 mm, 33/44 mm) and delivered via transseptal, transatrial or transapical routes (30 Fr). Its truncated cone has a low height profile (21 mm) that facilitates delivery and reduces risk of protrusion into the atrium or ventricle. Two rows of annular winglets anchor the bioprosthesis onto the mitral annulus. The first-in-human implantation was performed in October 2015 and satisfactory valve performance at eight months has been reported (NaviGate Cardiac Structures, Inc. First Navigate patient at 8 months shows excellent mitral valve function and has returned to work. Available at: https://www.navigatecsi.com/announcement/ first-navi-patient-at-8-months-shows-excellent-mitral-valve-
function-and-has-returned-to-work/).
CAISSON TMVR
The fully retrievable Caisson valve (Caisson Interventional LLC [now LivaNova], Maple Grove, MN, USA) is delivered via the transfemoral-transseptal approach using a 31 Fr delivery system. There are two components, a D-shaped, self-expanding nitinol anchor and a self-expanding, nitinol frame, trileaflet porcine pericardial valve (Williams M. Caisson: design and clinical trial updates. Presented at: Transcatheter Cardiovascular Therapeutics [TCT], Washington, DC, USA, 31 October 2016). The early feasibility PRELUDE trial (ClinicalTrials.gov identifier: NCT02768402) aims to enrol 20 patients.
Tricuspid valve devices (Figure 3)
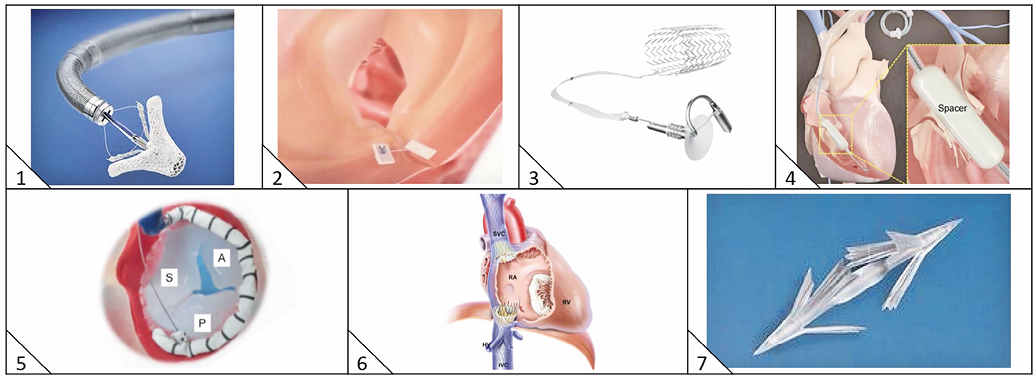
Figure 3. Transcatheter tricuspid valve repair devices. 1) MitraClip (Abbott Vascular, Santa Clara, CA, USA). 2) Trialign (Mitralign, Inc., Tewksbury, MA, USA). 3) TriCinch (4Tech Cardio Ireland Ltd., Galway, Ireland). 4) FORMA (Edwards Lifesciences, Irvine, CA, USA). 5) Cardioband TR (Edwards Lifesciences). 6) TricValve (P & F Products and Features Vertriebs GmbH, Vienna, Austria). 7) MIA (Micro Interventional Devices, Inc., Newtown, PA, USA).
`
Interventional strategies for tricuspid disease are still in their early stages. Anatomical challenges include the large annulus, paucity of valve/annular calcification, adjacency of the right coronary artery (RCA), and fragility of the valve tissue30. Thus far, percutaneous therapies used in humans reduce TR by: 1) occupying the regurgitant orifice area and providing a surface for leaflet coaptation (FORMA), 2) reducing annular dimensions (Mitralign and TriCinch), and 3) caval valve implantation to treat the reverse backflow associated with severe TR.
MITRACLIP®
The results of MitraClip (Abbott Vascular, Santa Clara, CA, USA) implantation in 64 patients with TR were recently described31 with success in 97% and no major complications. TR was reduced by at least one grade in 91% with significant reductions in effective regurgitant orifice area (0.9±0.3 cm² to 0.4±0.2 cm², p<0.001), vena contracta width (1.1±0.5 cm to 0.6±0.3 cm, p=0.001), and regurgitant volume (57.2±12.8 mL/beat to 30.8±6.9 mL/beat, p<0.001) accompanied by significant improvements in NYHA class and six-minute walk distance (165.9±102.5 m to 193.5±115.9 m, p=0.007).
FORMA
The FORMA system (Edwards Lifesciences) consists of a foam-filled polymer balloon (12 mm and 15 mm) spacer that occupies the regurgitant orifice area and provides a surface for leaflet coaptation and a six-pronged nitinol anchor that fixes the device at the right ventricular apex32.
The procedure is performed via left axillary venous access (20-24 Fr sheath) using a steerable catheter to deliver the rail system to the right ventricular apex. Under TOE guidance, the spacer (which has two radiopaque markers) is advanced over the rail to the tricuspid annular plane. The device is locked proximally and the excess rail coiled and placed within a subcutaneous pocket.
The first-in-human case series entailed seven patients with severe TR in NYHA Class II-IV (mean age 76±13 years, mean logistic EuroSCORE 25.7±17.4%)33. Device implantation was successful in all patients. At 30-day follow-up, all patients but one demonstrated improvement in NYHA class with significant reduction in TR severity (moderate in all patients) and peripheral oedema.
The Repair of Tricuspid Valve Regurgitation Using the Edwards TricuSPid TrAnsCatheter REpaiR System (SPACER) trial aims to assess the 30-day cardiac mortality of 75 patients undergoing the procedure compared with a literature-derived performance goal based on outcomes for high-risk tricuspid valve surgery.
TRIALIGN™
The Trialign device (Mitralign, Inc., Tewksbury, MA, USA) renders the tricuspid valve bicuspid by the placement of pledgeted sutures via transjugular access34. The system consists of a deflectable 8 Fr guide catheter for wire delivery, a pre-loaded pledget catheter with ventricular and atrial components, and a plication device to cinch and lock the sutures and maintain pledget position.
Access is via two 14 Fr jugular venous sheaths with a simultaneous right coronary guidewire as a fluoroscopic marker. The deflectable 8 Fr guide catheter is advanced into the right ventricle and articulated under the annulus to approach the posteroseptal commissure (under TOE guidance). A radiofrequency wire is advanced and positioned 2-5 mm from the base of the leaflet (hinge point) within the tricuspid annulus and directed towards the right atrium to avoid interference with the right coronary artery. The wire is snared from the jugular vein via the second sheath, externalised, then used to deliver the ventricular and atrial pledget components in sequence. These steps are then repeated on the anteroposterior commissure at a puncture site 24-28 mm away from the first pledget. Once positioned, the plication lock device is advanced over the sutures to cinch the two pledgets and plicate the annulus.
First-in-human implantation was reported in September 201435 and 30-day data from the early feasibility SCOUT trial were recently published36. The acute implantation success rate was 100% in 15 patients with NYHA Class ≥II and at least moderate functional TR. At 30 days, there were three single-pledget annular detachments without reintervention. In the remaining 12 patients, there were significant reductions in valve area (12.3±3.1 cm² to 11.3±2.7 cm², p=0.02) and effective regurgitant orifice area (0.51±0.18 cm² to 0.32±0.18 cm², p=0.02) accompanied by significant increase in LV stroke volume (63.6±17.9 ml to 71.5±25.7 ml, p=0.02).
TRICINCH™
The TriCinch system (4Tech Cardio Ireland Ltd., Galway, Ireland) consists of a corkscrew implant placed in the anterior tricuspid annulus (close to the anteroposterior commissure) and a self-expanding nitinol inferior vena caval stent37. Connection of these components by a Dacron band allows tensioning under TOE guidance to reduce annular dimensions and the severity of TR. First-in-human implantation was reported in 201538, and the Percutaneous Treatment of Tricuspid Valve Regurgitation With the TriCinch System (PREVENT) feasibility trial is ongoing.
CARDIOBAND® TR
The Cardioband (Edwards Lifesciences) direct annuloplasty device has also been used to address TR39 using a slightly modified delivery system to facilitate tricuspid annular implantation. The TRI-REPAIR CE mark trial is ongoing.
MIA™
The MIA annuloplasty implant (Micro Interventional Devices, Inc., Newtown, PA, USA) consists of ultra-low mass PolyCor™ anchors (Micro Interventional Devices, Inc.) and MyoLast™ implantable elastomer. First-in-human implantation was announced in December 2016 (http://www.prnewswire.com/news-releases/micro-interventional-devices-inc-announces-first-in-human-success-utilizing-mia-minimally-invasive-annuloplasty-technology-300381608.html), and the Study of Transcatheter Tricuspid Annular Repair (STTAR), a European multicentre safety and performance study, is ongoing.
Caval valve implantation
The first-in-human implantation of a custom-made, self-expanding valve in the inferior vena cava was described in 201140. The device was delivered via the right femoral vein and anchored at the cavo-atrial junction with valve alignment immediately above the hepatic inflow. Marked reduction in caval pressure was observed, enabling the patient to resume activities and ambulate without assistance within four days. Satisfactory device function was confirmed at eight-week follow-up with no recurrent right heart failure.
This concept is being explored in ongoing trials: 1) Heterotopic Implantation Of the Edwards-SAPIEN XT Transcatheter Valve in the Inferior Vena Cava for the Treatment of Severe Tricuspid Regurgitation (HOVER)41, 2) Treatment of Severe Secondary Tricuspid Regurgitation in Patients With Advanced Heart Failure With Caval Vein Implantation of the Edwards SAPIEN XT Valve (TRICAVAL). Pre-stenting is required to create a landing zone for valve deployment since the inferior vena cava is not calcified.
TRICVALVE™
The TricValves (P & F Products and Features Vertriebs GmbH, Vienna, Austria) are self-expanding, nitinol frame devices designed for bicaval valve implantation and do not require pre-stenting of the landing zone42. The superior vena cava device has a one-size valve diameter of 30 mm in its tubular portion but variable hip protrusion (up to 45 mm) to allow venous conformity. The inferior vena cava device is deployed with the jacketed portion in the right atrium and a waist in the hiatus of the diaphragm to permit hepatic venous drainage. Normalisation of liver function and improvement in NYHA Class (IV to II) has been reported in one patient at 24 months42.
Conclusions
Percutaneous intervention for mitral and tricuspid valve disease is evolving rapidly with multiple systems under evaluation. The majority of these are based upon well-established surgical techniques (modified to less invasive forms). It is hoped that they will provide a future alternative for the treatment of patients with high or prohibitive surgical risk.
Conflict of interest statement
P. Denti reports receiving consulting fees from Abbott, InnovHeart and 4Tech. S. Redwood reports receiving consulting fees from Edwards Lifesciences. B. Prendergast reports receiving consulting fees from Edwards Lifesciences and lecture fees from MVRx. The other authors have no conflicts of interest to declare.

