Abstract
The assessment and management of tricuspid valve (TV) disease have evolved substantially in recent years. Increased understanding of the long-term adverse consequences of severe tricuspid regurgitation (TR), along with continued advances in surgical and percutaneous techniques, has led to a renewed interest in the “forgotten valve”. The aim of this review is to explore the clinical and technical challenges related to transcatheter tricuspid valve treatment and to report the preliminary clinical experiences.
Introduction
The assessment and management of tricuspid valve (TV) disease have evolved substantially in recent years. Increased understanding of the long-term adverse consequences of severe tricuspid regurgitation (TR), along with continued advances in surgical and percutaneous techniques, has led to a renewed interest in the “forgotten valve”1,2.
Rarely, TR may be ascribed to a primary valve lesion; more frequently, a morphologically normal valve with annular dilatation and/or leaflet tethering due to right ventricular (RV) overload is encountered, so-called “functional TR” (FTR).
Surgical avoidance of TV repair was, for many years, easily accepted in patients with FTR secondary to left heart disease (LHD), on the basis of the incorrect belief that TR would disappear once the primary LHD had been treated. Over the past few years, many investigators have reported evidence in favour of a more aggressive surgical approach to FTR, and the most recent guidelines (Table 1) recommend surgical repair of concomitant TR during left valve surgery also in patients with tricuspid annular dilatation with non-severe TR3,4.
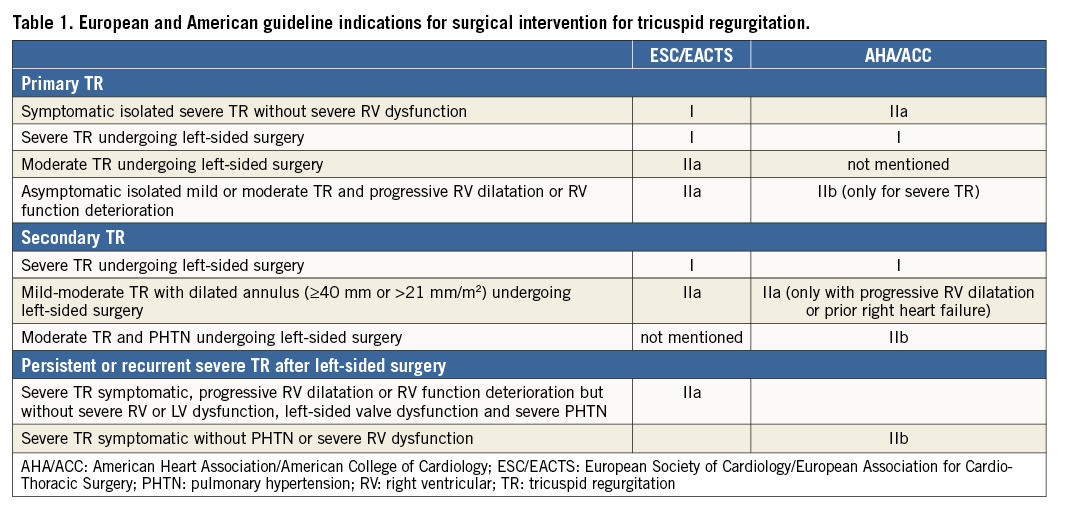
Although acceptable results have been reported5,6, in case of TR recurrence after left heart surgery, reoperation is usually carried out with very high morbidity and mortality (up to 25% in some series)7-9. This is largely due to the fact that typically these patients are managed medically for a long time and they are referred for surgery only when they develop severe incapacitating symptoms of right heart failure and organ dysfunction10.
Currently, moderate-to-severe TR affects up to 1.6 million patients in the USA, of whom, annually, only 8,000 undergo tricuspid surgery. Consequently, there is an extremely large number of untreated patients with significant TR11.
Percutaneous procedures have been emerging as an attractive alternative to surgery for patients deemed to be high-risk surgical candidates12. Different tricuspid transcatheter devices have been developed to treat TR. Some of them have already been used in humans, while others are still at the preclinical stage. Although feasibility has been shown with a number of devices, clinical and preclinical experiences with transcatheter TV therapies are still at a very preliminary stage and only few data are available to support any evidence of clinical efficacy. Today, physicians are facing up to several challenges, including proper patient selection, interventional timing, preprocedural and intraprocedural imaging, and how to report outcomes in a TV trial.
This review will explore the specific challenges of transcatheter TV interventions (TTVI) and the available technologies which are currently under evaluation.
Patient selection: which patients undergo TTVI today
Differently from transcatheter aortic valve implantation (TAVI) and transcatheter mitral valve repair (TMVR) in degenerative MR, in which the treatment of the valve disease can usually be curative, if timely and properly performed, TTVI has to be considered as a complementary tool in the context of a multilevel heart failure therapy.
At this stage of TTVI, most of the patients treated are inoperable end-stage heart failure patients, with RV dysfunction and severe comorbidities. In many cases the procedure is performed as a compassionate treatment. In this context, it can be extremely difficult to assess the potential clinical benefits of transcatheter TV therapies and to assess which patients could really benefit from an interventional strategy. Fluctuations of TR in response to loading conditions and medical therapies further compound this problem.
From a clinical standpoint, the main open issue regarding patient selection is to understand which patients could really benefit from a TTVI. Although it is well known that the presence of severe TR is independently associated with increased cardiac events and mortality in patients with congestive heart failure13,14, it is arguable whether correcting severe TR can provide a prognostic benefit in the presence of advanced heart failure. Indeed, it has been shown that, in patients with end-stage heart failure and NYHA Class III-IV, the presence of significant TR does not impact on survival, suggesting that in an advanced phase of the disease the prognostic impact of any eventual TV intervention could be minimal15. This underlines the importance of a proper interventional timing in order to avoid futile procedures. Severe RV failure and severe pulmonary hypertension should represent the most important contraindication to TTVI. However, since at the moment there are no data to define clearly which patients could benefit from an intervention, multidisciplinary Heart Team discussion is mandatory for each case, also taking into account frailty and life expectancy.
In order to understand the epidemiology of the patients who are treated today with TTVI and the dissemination of the different techniques, a large multicentre registry including patients treated with all the available devices is ongoing (TriValve registry: International multicenter Transcatheter Tricuspid Valve Therapies Registry). The preliminary results from the TriValve registry have recently been presented (Taramasso et al, International multisite Transcatheter Tricuspid Valve Therapies [TriValve] Registry: which patients undergo today transcatheter tricuspid repair? Presented at EuroPCR 2017, Paris, France). Among 106 patients included so far, the most used device was the MitraClip® (Abbott Vascular, Santa Clara, CA, USA) (55% of the cases), followed by annuloplasty techniques (Trialign™ [Mitralign Inc., Tewksbury, MA, USA] in 16% of the cases, TriCinch™ [4Tech Cardio Ltd, Galway Ireland] in 14% of the cases, Cardioband [Edwards Lifesciences, Irvine, CA, USA] in 5% of the cases). The FORMA spacer (Edwards Lifesciences) was used in 7% and caval valve implantation (CAVI) in 3% of the cases.
The larger diffusion of MitraClip in the tricuspid position compared to other devices can be explained by the wide availability of the device and by the familiarity of most operators with the mitral procedure. Therefore, several compassionate cases have been performed.
The clinical profile of the patients confirmed that today TTVI is performed significantly late during the course of the disease. Overall, patients are old (mean age 79 years) and presented with high surgical risk (EuroSCORE II 7.6%). Most patients (95%) are in advanced heart failure (NYHA Class III-IV, median NT-proBNP 2,253 pg/ml), mostly having already had acute RV decompensation (almost 60% of the patients were admitted at least once within the last six months, prior to TTVI due to RV failure). Mean duration of severe RV failure symptoms prior to the procedure was nine months (about 30% of the patients had ascites and more than 80% had peripheral oedema despite the maximal tolerated diuretic therapy at the time of TTVI).
The aetiology of TR is functional in more than 95% of the cases, with a “torrential” regurgitant jet (vena contracta [VC] more than 1 cm, effective regurgitant orifice area [EROA] more than 0.8 cm2). RV function was only mildly depressed according to the conventional definitions (tricuspid annular plane systolic excursion [TAPSE] 16 mm), with a moderate pulmonary hypertension (about 39 mmHg). TTVI was performed concomitantly to a transcatheter mitral intervention only in 32% of the cases.
Procedural success (defined as patient alive at the end of the procedure with the device successfully implanted and delivery system retrieved and a residual TR grade ≤2) was achieved in 62% of cases. Significant clinical improvement was observed at 30 days (NYHA class improvements, reduction of RV failure symptoms), with an all-cause mortality of 3.7%.
In general, the first report from the TriValve registry showed that multiple devices for transcatheter tricuspid valve therapy are currently being utilised in high-risk patients with functional aetiology and advanced TV disease. Initial results suggest that transcatheter tricuspid valve therapy is feasible and safe with different techniques, with a promising early clinical efficacy.
The anatomical challenges of TTVI: interventional perspectives
Although part of the knowledge that has been generated during the development of transcatheter mitral devices can be transferred to the TV, a deep understanding of the peculiar anatomy of the TV and of the right heart chambers, with differences and similarities between the two atrioventricular valves, is fundamental to overcome the specific challenges related to TTVI.
Similarly to the mitral valve, the TV is commonly approached antegradely. The most used approach is currently the transfemoral access, through the inferior vena cava (IVC) (MitraClip, Cardioband, TriCinch), while some devices are delivered through a transjugular approach (Trialign, FORMA). The absence of transseptal support and the short distance between the IVC orifice and the TV annulus represent major differences and challenges compared to MV interventions, resulting in a complete lack of stabilisation and difficult coaxiality.
The TV is located more anteriorly compared to the MV. As a consequence, intraprocedural transoesophageal echocardiography (TEE) guidance is particularly challenging in tricuspid procedures. In some circumstances, a combination of TEE, transthoracic echocardiography (TTE) and intracardiac echocardiography (ICE) is needed to obtain adequate imaging quality.
The major interventional issue related to the TV compared to the MV is its larger orifice (up to 9 cm2 area in normal condition, much larger in the presence of functional TR) (Figure 1). Therefore, a complete obliteration of the regurgitant area can be extremely cumbersome with the current repair devices. Similarly, it is easy to understand that a replacement device also has to be extremely large to cover the whole TV area and to seal it, especially in the absence of any type of annular or leaflet calcification, that is never observed in the tricuspid position.
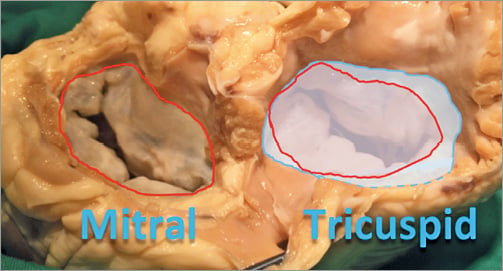
Figure 1. Comparison of the valve orifice of the mitral valve (red) and of the tricuspid valve (blue), showing the larger dimension of the second one.
The proximity of other cardiac structures has several implications in the tricuspid position. A peculiarity of the TV is the contiguity of the AV node, which is located in the proximity of the septal TV annulus, close to the anteroseptal commissure.
The risk of right coronary artery damage during interventional tricuspid procedures is mainly present in the annuloplasty procedure, and depends greatly on the coronary anatomy and dominance of the specific patients. Usually, the risk is high along almost all the anterior annulus and the entire posterior one.
The high chordal density in the TV subvalvular apparatus and the presence of RV trabeculae, together with more frail and thinner leaflets and chordal tissues compared to the mitral valve, make the risk of device impingement and tissue damage particularly high.
Diagnosis: the challenge of grading TR severity
Severe TR is associated with poor prognosis irrespective of age, left ventricular and right ventricular (RV) function and RV dimensions14. TTE and TEE represent the gold standard methods for preprocedural assessment of the TV. Clinically relevant measurements include: 1) an assessment of TR severity, 2) tricuspid annular diameter, 3) leaflet tenting measurements, and 4) RV size and function16.
TR severity assessment in particular represents a major challenge. Table 2 summarises the parameters most commonly used for grading the severity of TR, as described by the American Society of Echocardiography and the European Association of Cardiovascular Imaging guidelines17,18.
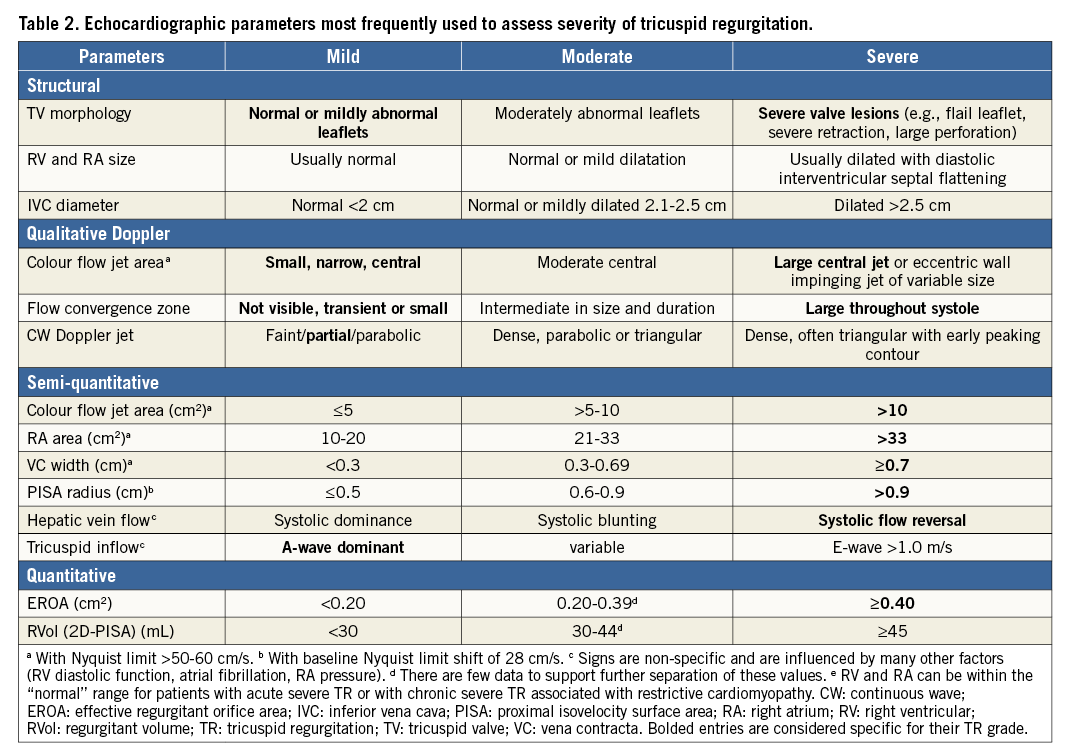
However, preliminary results from the TriValve registry showed that most of the patients treated today have really severe TR (often called massive or torrential TR). This is of particular importance for the evaluation of procedural results and residual TR, since it has been observed that a significant reduction in TR (although still severe according to the current guidelines) is associated with meaningful clinical benefits after TTVI19,20.
A number of methods can be used to perform quantitation of the TR severity. Although the proximal isovelocity surface area (PISA) method is simple and easy to perform21, the shape of the tricuspid regurgitant orifice is often elliptical22 or irregular, which may result in significant underestimation of the EROA by this method. More recently, 3D PISA has been used to quantify TR, analysing the largest convergence zone22. It has been shown that 3D PISA-derived EROA correlated well with 3D planimetered vena contracta area (VCA). Three-dimensional (3D) methods may help improve the accuracy of quantitative Doppler methods by planimetry of the VCA.
Procedural planning and intraprocedural guidance
Non-invasive imaging with echocardiography and computed tomography (CT) are crucial in selecting patients and assessing the feasibility of the procedure.
Since it offers a comprehensive assessment of the real 3D anatomy, CT allows the operator to assess the TV anatomy as well as the adjacent structures, including the proximity of the right coronary artery to the tricuspid annulus, the coronary sinus, the hepatic veins and vena cava (Figure 2). For some devices it is also crucial to assess the shape and dimension of the RV, including the distance between the RV apex and the annular plane (this is the case for the FORMA valve spacer). Moreover, CT angiography allows the operator to have a qualitative assessment of the tissue, which is particularly important for annulopasty devices which rely on anchor fixation, in order to identify the optimal target zone for the implant. Another feature of the CT scan is that it allows an easy and reliable prediction of the fluoroscopic working plane for each patient during the procedure, which are usually two, one en face view (normally LAO) and one view parallel to the annulus (normally RAO) (Figure 3).
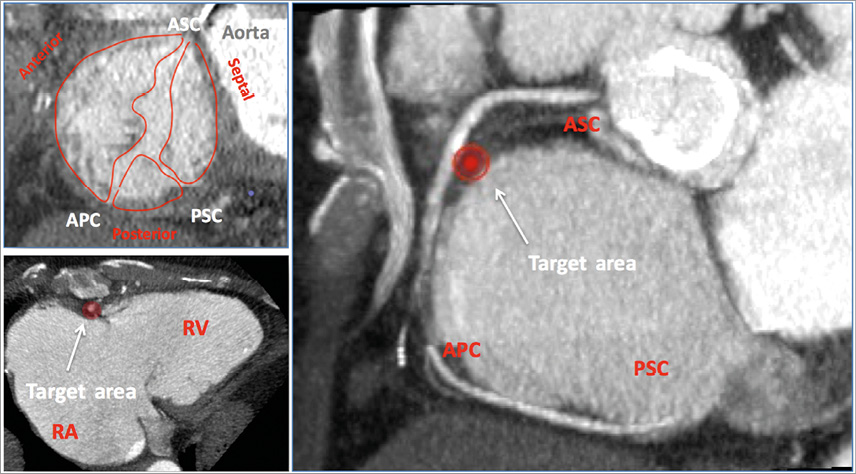
Figure 2. CT angio image of the tricuspid valve, showing long and short axis, leaflets and commissures. The red spot shows a spot far from the right coronary artery, which could be a potential target for an annuloplasty device. APC: anteroposterior commissure; ASC: anteroseptal commissure; RA: right atrium; RV: right ventricle; PSC: posteroseptal commissure
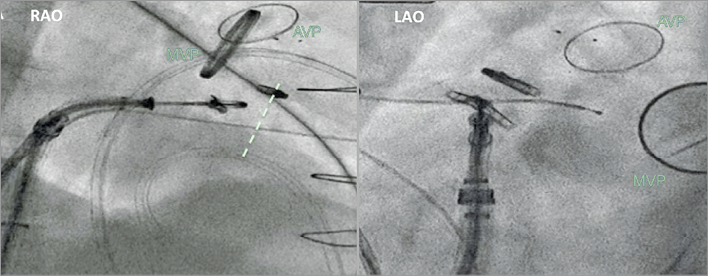
Figure 3. Fluoroscopic view used during transcatheter tricuspid treatment (in this case MitraClip). Right anterior oblique (RAO) view shows the long axis of the right ventricle and is used to assess the device trajectory; left anterior oblique (LAO) view shows the en face view of the tricuspid valve, which is used for navigation towards the therapeutic target. AVP: aortic valve prosthesis; MVP: mitral valve prosthesis. The dotted line indicates the tricuspid valve plane.
2D and 3D TEE are also essential imaging techniques in the assessment of patients with TR (Figure 4), in order to assess the mechanism and location of regurgitation, to quantify the annular dimensions and to assess the RV function and valve tethering.
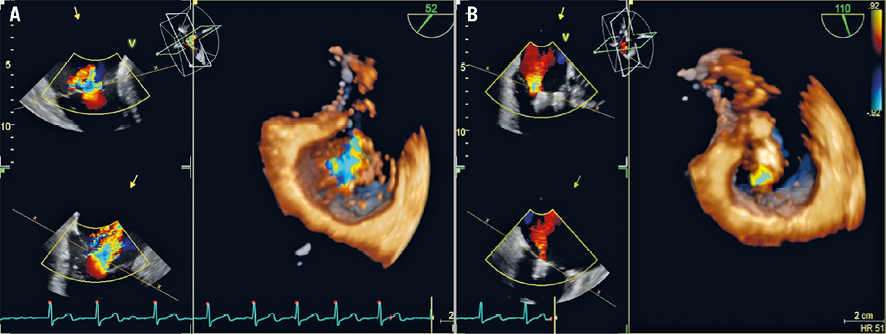
Figure 4. 2D and 3D TEE showing tricuspid regurgitation pre (A) and post (B) valve clipping.
In addition to echocardiography, cardiac magnetic resonance (CMR) imaging can provide detailed evaluation of the morphology and function of the right-side structures, in particular the TV and the RV. Steady 2D measurements of the annulus diameter, tethering height and lack of coaptation of leaflets can be obtained. The TR can be quantified in terms of vena contracta but also as regurgitant volume and fraction, in a similar way to mitral regurgitation. Moreover, CMR is currently the gold standard method for the assessment of RV morphology and function23,24.
Although preliminary experience has shown the feasibility of TV interventions, intraprocedural guidance remains a major issue, since in many patients the echocardiographic window for the TV is suboptimal and the procedural steps have not yet been standardised. 2D and 3D TEE play an essential role in guiding transcatheter TV interventions. In particular, procedural guidance with 3D TEE represents a fundamental tool to orient the navigation in the right atrium. However, in many patients the quality of intraprocedural guidance can often be suboptimal, as a consequence of the ventral location of the TV, mainly depending on patient variability and the individual echo window. In some patients, a combination of TTE and ICE may represent a useful complementary tool to guide the intervention. In particular, TTE is helpful in MitraClip procedures, in which a proper assessment of the leaflet insertion is mandatory to achieve procedural success. On the other hand, the use of ICE may be of value in the annuloplasty technique, since it is particularly accurate for visualising the leaflet-annulus hinge.
The use of the EchoNavigator® system (Philips Healthcare, Best, the Netherlands) may help to overcome the intrinsic limits of imaging guidance in TV interventions, since multimodality fusion imaging may define the target zone, increasing the chance of procedural success tremendously25. Additionally, it is of crucial importance to define a common nomenclature in order to standardise procedural 3D TEE guidance. A standardised nomenclature for 3D TEE navigation during TTVI has recently been proposed by Taramasso et al26.
Preliminary clinical and preclinical experiences with TTVI
The devices that have been developed for TTVI so far can be divided according to the therapeutic target being aimed at: leaflet devices, annuloplasty devices, heterotopic CAVI and transcatheter TV replacement (Figure 5).
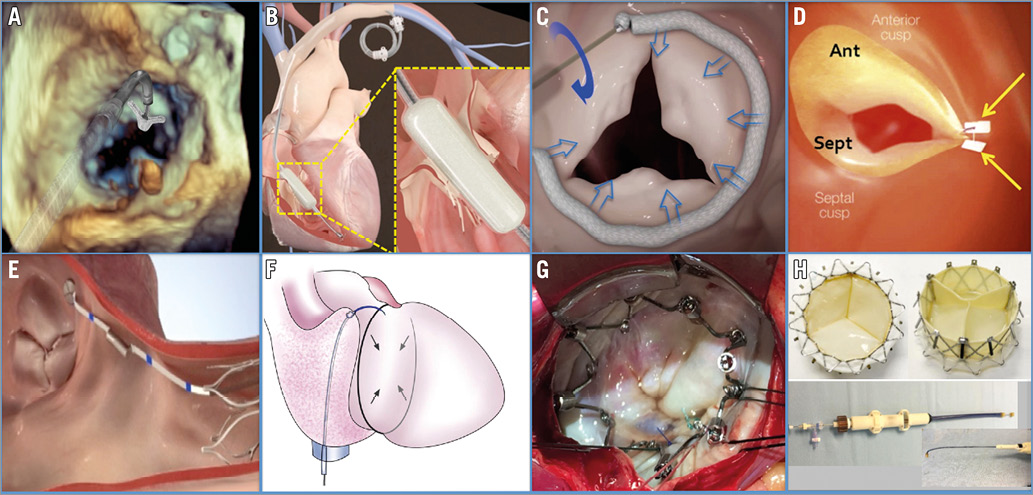
Figure 5. Tricuspid device parade: MitraClip (A), FORMA (B), Cardioband (C), Trialign (D), TriCinch (E), TRAIPTA (F), Millipede (G), Gate (prostheses and delivery system, H).
LEAFLET DEVICES
MITRACLIP IN TRICUSPID POSITION
The MitraClip system (Abbott Vascular) has been successfully used as an off-label procedure for treating severe tricuspid regurgitation in selected patients27,28. Although initially both the transjugular and transfemoral approaches were used, the transfemoral approach has become the preferred access today.
With about 400 compassionate cases performed worldwide, clipping of the TV is the TTVI technique with the widest clinical experience. However, procedural steps still have to be standardised and reported outcomes are still limited.
Two major issues have to be taken into consideration when transferring this technique from the mitral to the tricuspid valve: the difficulty of obtaining high-resolution images of the tricuspid valve, and the limited steering of the MitraClip system in the right atrium due to limited distance between the IVC orifice and the TV annular plane. Moreover, the absence of septal support makes the navigation in the RA particularly challenging.
Unlike the mitral position, in which the therapeutic target usually corresponds to the origin of the regurgitant jet, in functional TR the location of the TR is usually in the centre of the valve, in the context of a huge defect of capitation. Therefore, the rationale behind tricuspid clipping is to reduce the EROA by “closing” part of the valve, performing a sort of “annuloplasty with a leaflet device”. In most cases, this is obtained by closing the anteroseptal commissure, which is the easiest to target. A first clip is implanted close to the anteroseptal commissure, where the gap between the leaflets is minimum. Adjunctive clips are then implanted on the commissure line moving from the commissure towards the centre of the valve (“zipping by clipping”).
Nickenig et al recently reported promising 30-day outcomes in a series of 64 high-risk patients with severe FTR treated with tricuspid clipping29. The device was successfully deployed in 97% of the cases, with a very low rate of periprocedural complications. Thirty-day mortality was 5%.
In more than 50% of the cases, more than one clip was used. In about 80% of the cases the target was the anteroseptal commissure. A significant reduction in TR was reported (assessed with EROA, VCA and regurgitant volume). Clinical and functional improvement in terms of NYHA class and 6-minute walk test was also observed.
FORMA TRICUSPID SPACER
The FORMA repair system (Edwards Lifesciences) is a valve spacer, which has been designed to increase native leaflet coaptation surface by occupying the regurgitant orifice area20. It is composed of two parts: the spacer, which is positioned into the regurgitant orifice (two sizes are currently available, 12 mm and 15 mm), creating a platform for native leaflet coaptation, and a rail anchored at the right ventricle apex, which ensures device fixation. After a small surgical cut-down in the left subclavian region, the device is introduced through a venous subclavian access using a 24 Fr sheath. A right ventriculography is performed to locate the annular plane and the apical anchoring zone. A steerable delivery catheter is placed within the right ventricle for rail system delivering and the spacer is thereby deployed perpendicular to the TV, under TEE and fluoroscopic guidance. After the final positioning, the device is proximally locked at the subclavian region and the remaining rail length is placed into a subcutaneous pocket.
One of the major concerns regarding the FORMA device is the evaluation of residual TR after the procedure, since the presence of the spacer between the valve leaflets makes the accurate assessment and quantification of TR difficult.
The clinical outcomes (up to one year) of the first 18 patients treated with the FORMA device have recently been reported (Perlman G. Transcatheter Tricuspid Valve Repair with a New Transcatheter Coaptation Device for the Treatment of Severe Tricuspid Regurgitation - One-Year Clinical and Echocardiographic Results. Presented at EuroPCR 2017, Paris, France). Acute TR reduction of at least one grade was achieved in 89% of patients. Periprocedural complications included cardiac tamponade with conversion to open heart surgery (n=1), device dislocation (n=1), ventricular arrhythmia (n=1), and device thrombosis, solved with optimisation of anticoagulation therapy (n=1). Among patients having completed one-year follow-up, severe TR was reduced from 93% to 29%, with significant improvements in functional status. No deaths were observed at one-year follow-up. Reverse remodelling of the RV has also been observed.
ANNULOPLASTY DEVICES
CARDIOBAND
The Cardioband system (Edwards Lifesciences) is a direct annuloplasty device. It consists of a sutureless and adjustable surgical-like Dacron band, which is delivered through a fully percutaneous transfemoral approach and implanted on the atrial side of the valve annulus by means of multiple anchor elements. After the implantation of the Cardioband, the size of the implant can be reduced in a reversible fashion, in order to reduce the annular dimension and increase leaflet coaptation. The safety and feasibility of Cardioband implantation in the mitral position has been shown in high-risk patients with functional MR, with sustainable MR reduction and clinical benefit maintained up to six months30,31. The same concept has been successfully applied to the TV, performing a “surgical-like” undersized direct annuloplasty, which represents the gold standard surgical method for TV repair32. Preliminary results from the early feasibility trial have recently been presented (Kuck KH. Tricuspid Repair with the Edwards Cardioband System. Early Results of the TRI-Repair Study. Presented at EuroPCR 2017, Paris, France). Among 20 patients with severe FTR treated so far, a 27% reduction of septolateral tricuspid annular dimension has been reported. At 30 days, core lab adjudicated data (available only for a minority of patients) showed a reduction of VC and PISA EROA (29% and 44% compared to baseline, respectively), with significant improvement in quality of life and NYHA class. Two deaths occurred, both not device-related.
TRIALIGN™
The Trialign™ device (Mitralign, Inc., Tewksbury, MA, USA) (originally designed for the treatment of functional mitral regurgitation) allows the operator to perform a transcatheter bicuspidisation of the TV through a transvenous jugular approach, reproducing the surgical Kay bicuspidisation technique19. A steerable catheter is advanced across the TV in the RV under TEE and fluoroscopic guidance. An insulated radiofrequency wire is then advanced from the base of the leaflet and within the annulus, directed towards the right atrium (RA), corresponding to the anteroposterior commissure. Once the wire is through the annulus, a pledget delivery catheter is advanced over the wire from the RA across the annulus to the RV. The steps are then repeated on the opposite anatomic site of the posterior commissure. A dedicated plication lock device is used to bring the two pledgeted sutures together, plicating the annulus and effectively bicuspidising the TV. One or multiple pairs of pledgets can be implanted, depending on the severity of annular dilatation.
The SCOUT I feasibility trial (Early feasibility of a Percutaneous Tricuspid Valve Annuloplasty System for Symptomatic ChrOnic FUnctional Tricuspid regurgitation) included 15 patients with severe TR who underwent tricuspid annuloplasty with the Trialign19. The device was successfully implanted in all cases, with only one procedural complication (new right coronary stenosis requiring coronary stenting). At 30-day follow-up, survival was 100%, with a technical success rate of 80% (three cases of single pledget dehiscence). No major adverse events were observed. Significant reduction in the tricuspid annular area and the EROA, as well as improvement in functional class and quality of life, was maintained up to six months.
TRICINCH™
The TriCinch (4Tech Cardio Ireland Ltd., Galway, Ireland) is a catheter-based device designed to perform tricuspid annular cinching, in order to reduce anteroseptal annular dimension, improve leaflet coaptation and reduce TR33,34. Through a transfemoral access, the tip of a steerable catheter is positioned between the anteroposterior commissure and the mid-anterior annulus; there, a corkscrew element is implanted on the tricuspid annulus. Right coronary artery angiography is performed to exclude coronary damage. Once the corkscrew is secured, the delivery system is retrieved and a self-expanding nitinol stent is introduced over the wire and coupled to the implant. The whole system is then tensioned to reshape the TV and to increase the leaflet coaptation, under live echo guidance. Finally, the stent is deployed in the inferior vena cava (IVC) to maintain the tension applied. Enrolment of patients in the early feasibility trial (PREVENT: Percutaneous Treatment of Tricuspid Valve Regurgitation with the TriCinch System) has recently been completed in Europe.
Among 24 patients treated, the device was successfully implanted in 18 (85%) patients. Reasons for non-completed procedures were intraprocedural complications (n=2, hemopericardium) or annulus anchor detachment (n=4), without severe adverse events. No patient had died at 30 days, and a reduction of at least one grade in TR was observed in 94% of the cases. During one year of follow-up, four late anchor detachments were observed (17%). In order to overcome this issue, a second-generation device is currently under preclinical evaluation (Denti P. Preclinical experience with new percutaneous tricuspid valve repair system. Presented at EuroPCR 2017, Paris, France): the corkscrew has been replaced by a coil anchor element which is implanted in the pericardial space, allowing more tensioning, minimising the risk of detachment.
TRAIPTA CONCEPT
The TRAIPTA concept (transatrial intrapericardial tricuspid annuloplasty) uses a different approach. Pericardial access is obtained puncturing the RA appendage, after transfemoral venous access. A circumferential implant, which exerts compressive force over the annulus, is delivered along the AV groove within the pericardial space. Tension on the implant is then adjusted interactively to modify tricuspid annular geometry and therefore reduce TR. The RA puncture is then sealed, using off-the-shelf nitinol closure devices. The device has been successfully implanted in 16 swine with functional TR, leading to significant tricuspid valve geometry changes (reduction in annular area and perimeter, and increase in coaptation length)35. A new TRAIPTA device for human testing is currently being developed.
The major limitation of this approach is that it requires a free pericardial space. Therefore, it cannot be used in patients with previous heart surgery. This aspect may reduce the adoption of the TRAIPTA device, since many patients who could benefit from TTVI present with late recurrence of FTR after mitral surgery. Compression of coronary arteries may represent another issue of this approach, and should be assessed before the final release.
MILLIPEDE
The Millipede system (Millipede, LLC, Ann Arbor, MI, USA) is a fully repositionable and retrievable complete ring, which can be implanted surgically or transcatheter, on the atrial side of the native tricuspid annulus in order to restore its shape and diameter. Corkscrew-shaped anchors attach the ring to the fibrous annulus. A first-in-man implant in the mitral position was performed surgically in 2015. So far, among nine patients who have undergone mitral valve repair with the Millipede system, two of them have had concomitant tricuspid valve repair, showing tricuspid diameter reductions of 42-45%, with no post-procedural residual TR (Rogers J. Transcatheter tricuspid valve therapies: Millipede. Presented at TCT 2016, Washington, DC, USA).
CAVAL VALVE IMPLANTATION (CAVI)
An alternative approach to the percutaneous treatment of TV is to implant a transcatheter prosthesis in the IVC (single valve approach) or in combination with a superior vena cava (SVC) valve (dual valve) to prevent damage to the liver and other organs. The rationale of this procedure is to reduce hepatic, abdominal, and peripheral venous congestion leading to amelioration of right heart failure. Since the prostheses are implanted in a low-pressure venous system, lifelong anticoagulation would probably be required. In the presence of advanced RV dysfunction, the single-valve approach seems to be safer compared with the dual-valve approach since it increases RV preload less. However, no data are available on which should be the optimal treatment (single vs. dual valve). Different devices have been implanted using this approach and there is no consensus on what would be the ideal CAVI prosthesis. A dedicated, self-expanding nitinol prosthesis named the TricValve (P & F Products & Features Vertriebs GmbH, Vienna, Austria in cooperation with Braile Biomedica, Sao José do Rio Preto, Brazil) has been specifically developed for the venous low-pressure circulation and to seal the large caval anatomy. Two bioprosthetic valves are available, with sizes ranging from 28 to 43 mm (up to 38 mm for the SVC, 43 mm for the IVC).
Results from a multicentre registry including 23 patients have recently been reported (Lauten A. Caval Interventional Treatment Option for Valve Implantation [CAVI] as interventional treatment option for severe TR. Presented at EuroPCR 2017, Paris, France): 19 patients were treated with single valve implantation (IVC only), while four had bicaval implantation. Both balloon-expandable and self-expanding valves were used. When self-expanding valves were used, different techniques of IVC landing-zone downsizing were applied (including pre-stenting or surgical banding). Implant was feasible in all patients. Thirty-day survival was 82.6% (n=19); long-term survival was reported in eight patients with an overall median survival of 98 days. Early valve migration requiring surgical intervention occurred in one patient.
TRANSCATHETER TRICUSPID VALVE REPLACEMENT
Transcatheter tricuspid valve implantation in the context of a degenerated surgical bioprosthesis (valve-in-valve) or after tricuspid annuloplasty (valve-in-ring) has been performed using different devices and accesses36,37.
The Gate™ self-expanding tricuspid atrioventricular valved stent (NaviGate Cardiac Structures Inc., Laguna Hills, CA, USA) is the only available device that has been used to perform transcatheter tricuspid valve replacement in humans in the native tricuspid valve. It is a nitinol tapered stent with three xenogeneic pericardial leaflets, and annular winglets enable fixation of the annulus and the leaflets, avoiding protrusion into the adjacent chambers. The different sizes available are 30/40 mm, 33/44 mm and 36/48 mm.
Two compassionate implants have been performed at the Cleveland Clinic, one through the transatrial and one through the transjugular approach, with mild residual paravalvular TR and both patients alive up to five months after the implantation (Navia JL. First-in-Man Experience of a Novel Transcatheter Tricuspid Valve Replacement in a Patient with Severe Tricuspid Valve Regurgitation. Presented at EuroPCR 2017, Paris, France).
Reporting outcomes after TTVI: towards T-VARC
The analysis of preliminary results from the early feasibility trial with the different devices raises the issue of how to report outcomes after TTVI in the future. It is extremely important to have a precise quantification of the entity of TR before and after any intervention. In fact, patients consistently report improvements in quality of life measures despite only modest reductions in TR according to the conventional definitions. Given the discordance between TR reduction and clinical improvement, it is evident that we cannot use definitions of procedural success for the TV that have been used for the aortic and mitral valves. Moreover, a reduction from torrential to severe TR may still be beneficial for the patient by reducing anasarca and intestinal oedema and improving the efficacy of medical therapy in patients with right heart failure. This observation raises another issue in the burgeoning field of interventional TV therapies - the need to define appropriate outcomes in this patient population and develop standard definitions regarding how to evaluate and report these outcomes.
Conclusions
Although TTVI is still at a really early stage, initial results have shown feasibility and safety with different techniques and devices. Although efficacy has still to be proved, initial results have shown promising clinical improvements. In the near future, several clinical and technical open issues have to be addressed. Defining appropriate outcomes in this patient population and developing standard definitions regarding how to evaluate and report procedural and patient success will be a crucial step towards assessing the efficacy of TTVI compared to optimal medical therapy. Moreover, at present, patient selection, anatomical eligibility and optimal procedural timing remain to be defined.
Conflict of interest statement
M. Taramasso is a consultant for Abbott Vascular and 4Tech. F. Maisano is a consultant for Abbott Vascular, Edwards Lifesciences and Medtronic, and is a co-founder of 4Tech.

