Abstract
Transcatheter tricuspid valve repair/replacement is an emerging therapy for patients with symptomatic severe tricuspid regurgitation who are deemed inoperable. Accurate knowledge of the anatomy of the tricuspid valve and right ventricle is key to developing transcatheter techniques. In addition, it is important to understand the mechanistic concept of transcatheter tricuspid valve repair/replacement in order to select the patients who may benefit from it. The severity and mechanism of tricuspid regurgitation, right ventricular function, dimensions of the caval veins and the course of the right coronary artery in relation to the atrioventricular groove are important aspects to be evaluated before embarking on these procedures. The present article reviews current advances in transcatheter approaches for significant tricuspid regurgitation and the role of imaging modalities to characterise the anatomy of the tricuspid valve and right ventricle as well as the underlying pathophysiology of tricuspid regurgitation.
Introduction
A better understanding of the complex three-dimensional (3D) structure of the tricuspid valve and the right ventricle has facilitated the development of novel transcatheter therapies for tricuspid regurgitation (TR), a frequent valvular heart disease that has been largely ignored. Whilst a trivial to mild degree of TR is frequently observed and considered benign (in the absence of pulmonary hypertension or heart failure)1,2, the prevalence of significant (moderate and severe) TR may reach 16% in selected populations3. Primary TR occurs due to organic damage of the tricuspid valve (endocarditis, leaflet prolapse, chordal rupture, rheumatic valve disease) and is rather infrequent (8-10%)4. In contrast, secondary or functional TR is more frequently observed and is characterised by anatomically preserved leaflets. It occurs due to annulus dilatation and right ventricular (RV) remodelling, which is commonly caused by left ventricular failure due to myocardial or valvular diseases1,4,5.
Significant TR, even if isolated, is associated with RV dysfunction at follow-up and impaired prognosis3,6,7. Current guidelines recommend surgical intervention for symptomatic severe TR (class I) and in patients with moderate TR or mild TR with severe tricuspid annulus dilatation (≥40 mm on echocardiography) undergoing left-sided valve surgery (class IIa)2,5. The concept that mild functional TR should disappear after correction of the primary left-sided valvular disease problem has been countered by several studies showing that patients with mild TR left untreated at the time of the left-sided valve surgery were at risk of developing significant TR at follow-up5,8. These patients pose a therapeutic challenge and are not routinely referred for surgery since reoperation for significant TR is associated with 10-25% in-hospital mortality5. As an alternative to open heart surgery, percutaneous technologies may provide a less invasive treatment option.
Current advances in transcatheter approaches for significant TR and the role of imaging modalities to characterise the anatomy of the tricuspid valve and right ventricle and the underlying pathophysiology of secondary or functional TR will be reviewed in this article.
Transcatheter tricuspid valve repair/replacement techniques (Online Appendix 1)
The characteristics and mechanism of action of the different available devices are summarised in this section.
HETEROTOPIC IMPLANTATION OF CAVAL VALVES
Lauten et al reported the successful implantation of a self-expandable tubular stent tailored to the dimensions of the inferior vena cava at the landing zone9. The stent frame was manufactured of nitinol and shaped to ensure appropriate apposition at the cavoatrial junction. The procedure was performed through the transfemoral access and guided with a combination of fluoroscopy and transoesophageal echocardiography (TEE). Significant reductions in inferior vena cava pressures were reported (from 29/19 mmHg to 19/12 mmHg). In addition, commercially available balloon-expandable valves (29 mm SAPIEN XT; Edwards Lifesciences, Irvine, CA, USA) have been successfully implanted in the inferior and superior vena cava10,11. To ensure a stable position for the device, the landing zone is prepared with self-expandable stents which reduce the lumen in the inferior and superior vena cava segments downstream and upstream of the right atrium. Pre-procedural demonstration of severe TR and systolic flow reversal in the inferior vena cava is important to guarantee good function of the caval valve. In addition, accurate measurement of the inferior and superior vena cava diameters and the distance between the landing zone and the hepatic veins is crucial to select appropriate device size and to avoid obstruction of the hepatic veins by the device. Assessment of RV function is pivotal. This procedure leads to ventricularisation of the right atrium with subsequent increase in right atrial pressure and RV preload which may cause further deterioration of RV failure in patients with impaired RV function and pulmonary hypertension. Finally, the presence of pacemaker leads precludes appropriate anchoring of the device in the superior vena cava.
DIRECT TRICUSPID VALVE ANNULOPLASTY
Using the Mitralign system (Mitralign Inc., Tewksbury, MA, USA), Schofer et al reported the first-in-man transcatheter tricuspid valve repair12. Originally designed for the mitral valve, this system uses radiofrequency wires that cross the valve annulus and two pledgets that are passed over the wires and connected with a drawstring. By plicating the string, the pledgets are approximated to each other, thereby reducing the perimeter of the valvular annulus. For tricuspid valve annuloplasty, a deflectable catheter introduced through the jugular vein can be used to manoeuvre and place the radiofrequency wires and the pledget sutures at the tricuspid annulus, at the level of the antero- and the septo-posterior commissures. Using a dedicated plication lock device, the sutures are approximated resulting in bicuspidalisation of the tricuspid valve and reduction of the tricuspid valve annulus dimensions and TR. In addition, Maisano and co-workers presented the preliminary results of the TriCinch System (4Tech Cardio Ltd., Galway, Ireland) at the 2015 EuroPCR meeting13. This device consists of a corkscrew anchor connected through a Dacron band to a self-expandable stent which is implanted transfemorally under fluoroscopic and echocardiographic guidance. The corkscrew anchor is positioned at the tricuspid annulus supporting the anterior leaflet (between the antero-septal and antero-posterior commissures). By applying tension to the system, the tricuspid annulus is reshaped, improving leaflet coaptation and reducing TR severity. To maintain the remodelling applied, the stent is deployed in the inferior vena cava (Online Figure 1). The results of the ongoing Percutaneous Treatment of Tricuspid Valve Regurgitation With the TriCinch System (PREVENT) trial (NCT02098200) will provide further evidence on the safety and efficacy of this procedure14.
In both procedures, assessment of the course of the right coronary artery in relation to the tricuspid valve annulus should be performed before the procedure, as well as monitoring of the patency of the artery during the procedure.
INDIRECT TRICUSPID VALVE ANNULOPLASTY
The transcatheter transatrial intrapericardial tricuspid annuloplasty (TRAIPTA) system consists of an interrupted 0.035 inch nitinol wire, preshaped into a self-expandable loop which opens inside the pericardium encircling the heart along the atrioventricular groove15. In a swine model, Rogers et al demonstrated the efficacy of this system in reducing TR severity15. After entering the pericardium via a puncture through the right atrial appendage, the nitinol wire is delivered and tightened, thereby reducing the tricuspid valve annulus and diminishing the TR severity. Multi-detector computed tomography (MDCT) data on patients with RV dilatation demonstrated that the right coronary artery crosses the projected course of the TRAIPTA device15. To avoid impingement of the right coronary artery, bridge-like elements have been developed to direct the compressive force away from the coronary artery. The safety and efficacy of this device in clinical practice need further evaluation.
Multimodality imaging in transcatheter tricuspid valve repair/replacement: key imaging steps for pre-procedural planning (Online Appendix 2)
Characterisation of the anatomy of the right ventricle and tricuspid valve (Online Figure 2) is the first step in the evaluation of patients with functional TR who may be candidates for transcatheter tricuspid valve repair/replacement. In addition, the following aspects should be accurately evaluated:
1. Severity of TR.
Colour Doppler transthoracic echocardiography is the imaging technique of choice to analyse the severity of TR. The presence of central large regurgitant jets extending deep into the right atrium in multiple acoustic windows indicates severe TR. However, eccentric swirling jets which reach the posterior wall of the right atrium also suggest severe TR. With continuous wave Doppler, the spectral signal of severe TR is dense and triangular with an early peak velocity (<2 m/s), indicating massive TR. Semiquantitative and quantitative parameters of TR are recommended to define better the severity of TR1. The measurement of the vena contracta width (the smallest diameter of the regurgitant jet as it leaves the valve) in the apical four-chamber view is one of those parameters. A vena contracta width ≥7 mm indicates severe TR (Figure 1). In addition, the flow convergence or the proximal isovelocity surface area (PISA) method permits calculation of the effective regurgitant area and the regurgitant volume. This method assumes a circular regurgitant orifice, which is not often the case in TR. Therefore, this method is less feasible and accurate in TR, as compared to mitral regurgitation. An effective regurgitant orifice area ≥40 mm2 or a regurgitant volume ≥45 mL/beat indicates severe TR. Finally, the presence of systolic flow reversal in the hepatic veins, as assessed with pulsed wave Doppler, also indicates severe TR. The role of 3D colour Doppler echocardiography to quantify the regurgitant volume has not been extensively evaluated. Besides echocardiography, magnetic resonance imaging has been shown to assess regurgitant volume reliably in TR16.
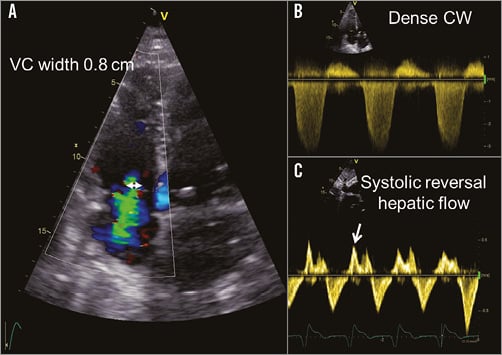
Figure 1. Assessment of tricuspid regurgitation severity. The measurement of the vena contracta (VC) in the apical four-chamber view is a recommended semiquantitative parameter to assess the severity of the TR (A, double arrowhead). The presence of a triangular dense envelope of the spectral signal of the tricuspid regurgitant jet on continuous wave (CW) Doppler echocardiography indicates severe TR (B). The presence of systolic flow reversal in the hepatic veins on pulsed wave Doppler echocardiography also indicates severe TR (C, arrow).
2. Tricuspid valve anatomy and leaflet mobility.
Tricuspid annulus dilatation is the most frequent mechanism of TR. On 2D transthoracic echocardiography, a tricuspid valve annulus ≥40 mm defines significant dilatation. Using MDCT multiplanar reformation planes, the cross-sectional area and eccentricity of the tricuspid annulus can be assessed, providing more accurate measurements of the tricuspid annulus compared with 2D transthoracic echocardiography (Figure 2)17. In addition, the presence of restrictive movement of the tricuspid leaflets suggests the presence of significant leaflet tethering (secondary to excessive RV dilatation), entrapment of the leaflets (due to pacemaker leads) or primary damage of the leaflets (carcinoid syndrome). In contrast, excessive motion of the leaflets (prolapse) is due to primary damage of the leaflets (endocarditis, perforation) or subvalvular apparatus (chordal rupture). Heterotopic implantation of caval valves may help to reduce the symptoms of severe TR, regardless of the underlying mechanism. In contrast, direct or indirect tricuspid annuloplasty techniques are more suited for functional TR caused by annular dilatation.
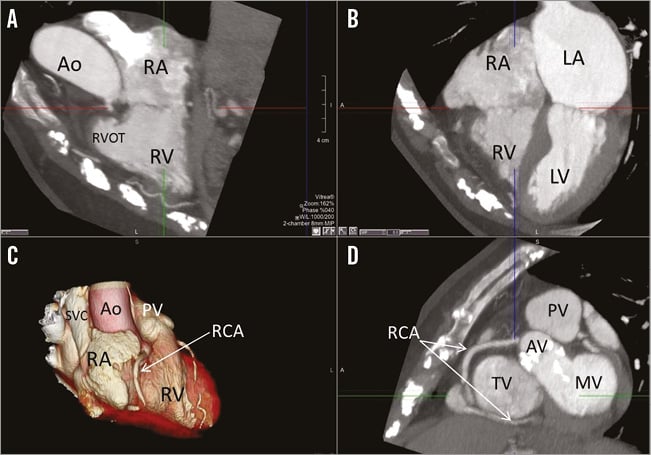
Figure 2. Multi-detector computed tomography to assess the tricuspid annulus dimensions and the anatomical relationship between the right coronary artery and the atrioventricular groove. From the two-chamber (A) and four-chamber (B) views of the right ventricle, an orthogonal plane is set at the level of the tricuspid annulus providing the cross-sectional plane of the tricuspid annulus (D). C) 3D volume rendering of the right side of the heart. In panels C and D, the right coronary artery (RCA) is demarcated indicating its course along the atrioventricular groove. Note the short distance between the RCA and the tricuspid annulus at the level of the antero-septal and antero-posterior commissures (arrows). Ao: aorta; AV: aortic valve; LA: left atrium; LV: left ventricle; MV: mitral valve; PV: pulmonary valve; RA: right atrium; RV: right ventricle; RVOT: right ventricular outflow tract; SVC: superior vena cava; TV: tricuspid valve
3. RV systolic function.
This is an important parameter to assess in patients who are candidates for heterotopic transcatheter caval valves. These procedures lead to a ventricularisation of the right atrium with a consequent increase in RV preload. In patients with impaired RV systolic function this increase in preload may further impair RV systolic function and worsen the prognosis. Two-dimensional transthoracic echocardiography is the imaging technique most available to assess RV function. RV fractional area change <35% and tricuspid annulus plane systolic excursion (TAPSE) index <16 mm indicate RV systolic dysfunction18. Other parameters based on tissue Doppler imaging and speckle tracking echocardiography have provided additional functional parameters which reflect the performance of the RV myocardium. However, the characteristic 3D shape of the RV is better assessed with 3D imaging techniques. Normal reference values of RV volumes and function with 3D transthoracic echocardiography have been reported19. Magnetic resonance imaging is considered the reference standard for assessment of RV volumes and function18.
4. Diameter of the inferior and superior vena cava.
For the heterotopic implantation of transcatheter valves in the vena cava, MDCT may be used to evaluate the dimensions of the inferior vena cava from the first hepatic vein until the transition level to the right atrium11. To avoid obstruction of the hepatic vein it is recommended to implant the valve at least 1 cm above the first hepatic vein11. As the inferior vena cava becomes wider from the hepatic vein to the cavoatrial junction, MDCT data may help in the decision to implant an additional stent at the cavoatrial junction to facilitate a more homogenous landing of the caval valve11.
5. The course of the right coronary artery in relation to the atrioventricular groove.
The use of anchor devices on the tricuspid annulus or indirect annuloplasty systems requires accurate assessment of the course of the right coronary artery in relation to the atrioventricular groove. MDCT provides high spatial resolution images to assess this aspect (Figure 2).
In summary, transcatheter tricuspid valve repair/replacement is a developing technique in which a thorough knowledge of the anatomy of the tricuspid valve and RV and an accurate assessment of the TR severity and mechanism are crucial to select the patients who may be candidates for these procedures. Echocardiography, MDCT and MRI can provide the information needed.
Conflict of interest statement
The Department of Cardiology receives research grants from Edwards Lifesciences, Biotronik, Medtronic and Boston Scientific. V. Delgado has received speaker fees from Abbott Vascular. The other authors have no conflicts of interest to declare.
Online data supplement
Appendix 1. Additional information on transcatheter tricuspid valve repair/replacement techniques.
Following the success of transcatheter therapy for aortic stenosis, mitral regurgitation and failing bioprosthesis, as well as tricuspid and mitral ring annuloplasty, experiences on transcatheter treatment of native TR are accumulating9-12,15. Initially, the procedural approach was classified based on the site of implantation of the transcatheter valve as orthotopic, if placed within the tricuspid valve annulus, and heterotopic, when placed in the inferior and/or superior vena cava at the level of the cavoatrial junction. The anatomic, geometric and dynamic characteristics of the tricuspid valve annulus (triangular and non-planar shape, highly dynamic during the cardiac cycle and more flexible than the mitral annulus) challenge the development of devices that will stabilise and fixate the transcatheter valve in an orthotopic position. In contrast, the heterotopic approach has shown that implantation of valve devices in caval veins results in significant improvement in right heart haemodynamics and reductions in TR9-11. Meanwhile, direct and indirect tricuspid valve annuloplasty procedures have been developed, and the first experimental and clinical experiences have been reported12,13,15.
Appendix 2. Additional information on multimodality imaging in transcatheter tricuspid valve repair/replacement: key imaging steps for pre-procedural planning.
The right ventricle is characterised by a complex 3D geometry with a triangular shape when viewed laterally and a crescent shape in the cross-sectional view. Moreover, the right ventricle is frequently divided into three functional subunits: the inflow region (which contains the tricuspid valve), the trabeculated muscular apex, and the outflow region or infundibulum (which includes the pulmonic valve). The lack of continuation between the tricuspid and the pulmonic valve which are separated by the muscular ventriculo-infundibular fold is one of the morphological characteristics that differentiate the right ventricle from the left ventricle. In addition, the septal aspect of the right ventricle contains several characteristic anatomical landmarks: the septomarginal trabeculation, a Y-shaped muscular band that embraces the ventriculo-infundibular fold and gives rise to the medial papillary muscle, and the moderator band which originates from the body of the septomarginal trabeculation and crosses the ventricular cavity carrying one fascicle of the right bundle branch. The tricuspid valve consists of three leaflets attached to an asymmetrical saddle-shaped tricuspid annulus with the lowest and highest points in a mediolateral and anteroposterior direction, respectively (Online Figure 2)4. The anterior leaflet is the largest and it is supported by the medial papillary muscle which originates from the moderator band. The posterior leaflet is the most inferior and is supported by several papillary muscles that are attached to the diaphragmatic wall of the right ventricle. Finally, the septal leaflet is another anatomical landmark that permits identification of the right ventricle. This leaflet is the smallest, has numerous tendinous cords attached to the ventricular septum and its hinge line is closer to the ventricular apex. The septal part of the tricuspid annulus is similar to the intertrigonal section of the mitral annulus whereas the other two parts are more flexible, explaining the characteristic dilatation in the anteroposterior direction and causing malcoaptation of the leaflets4,20. In addition, chordae arising from the RV wall, septal wall and moderator band may further explain leaflet tethering in the presence of RV dilatation4.
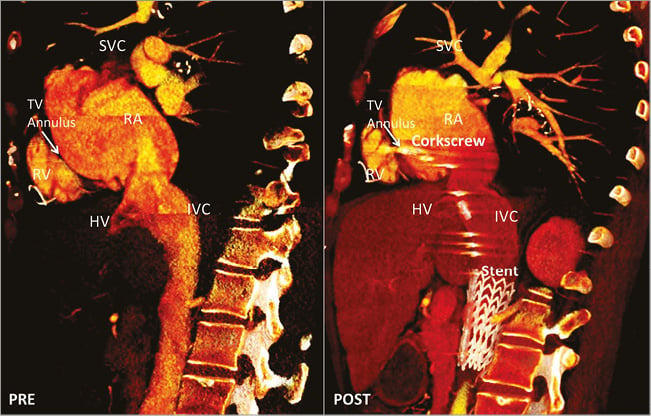
Online Figure 1. Multidetector-row computed tomography of the right heart and inferior vena cava before (PRE) and after (POST) implantation of the TriCinch System. On the sagittal view, the cavoatrial junction and the position of the hepatic veins relative to the landing zone can be visualised. The corkscrew is implanted at the level of the anterior part of the tricuspid annulus and the stent is deployed in the inferior vena cava to provide tension to the system and reduce the anteroposterior diameter of the annulus. Courtesy of 4Tech Cardio Ltd., Galway, Ireland.
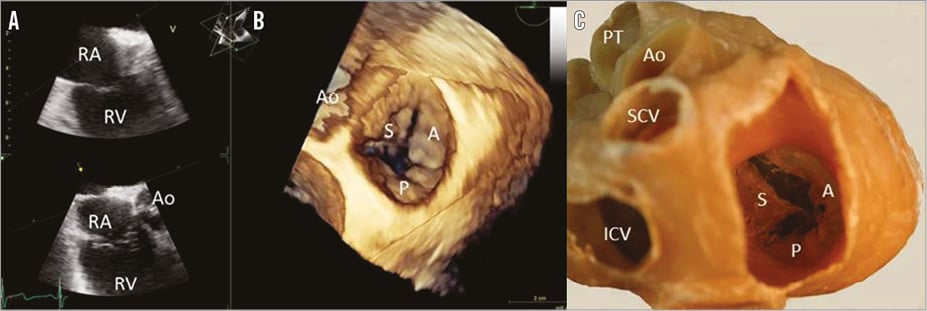
Online Figure 2. Anatomy of the tricuspid valve. A) Two orthogonal views of the tricuspid valve on transoesophageal echocardiography to ensure inclusion within the sector view of the entire valve. B) 3D reconstruction of the tricuspid valve viewed from the right atrium with transoesophageal echocardiography. C) Dissection of the cardiac base and the atrial visualisation of the tricuspid valve (courtesy of Dr Monique Jongbloed, Leiden University Medical Center, Leiden, The Netherlands). A: anterior leaflet; Ao: aorta; ICV: inferior vena cava; P: posterior leaflet; PT: pulmonary trunk; RA: right atrium; RV: right ventricle; SCV: superior vena cava

