
Abstract
Nowadays, tricuspid regurgitation (TR) is a widespread condition and this has led to a not-unexpected interest in the “forgotten valve”. Medical treatment and/or a surgical approach remain the gold standard for this valve disorder, although these approaches are often limited. In the era of transcatheter therapies, the primary interest for less invasive percutaneous approaches targeted to the treatment of TR can thus find a clear rationale for its evolution. The aim of this review is to explore the different transcatheter techniques for TR that are available or under study, and also to report on preliminary clinical results worldwide.
Abbreviations
CAVI: caval valve implantation
TR: tricuspid regurgitation
Introduction
Moderate-to-severe tricuspid regurgitation (TR) is a prevalent condition independently associated with significant adverse outcome in terms of morbidity and mortality1,2. It is seldom ascribed to an isolated entity and its prevalence is rising, especially among patients who suffer from other valve disorders, in particular left-sided valve diseases3. The functional or secondary form of TR is the most common entity in Western countries, mainly caused by an increase in left atrial pressure followed by a decrease in pulmonary vascular compliance and an increase in pulmonary vascular resistance. These changes in the pulmonary vascular bed may result in dilatation of the right ventricle and consequently of the tricuspid annulus, with resulting leaflet tethering and malcoaptation4 (Figure 1). Right ventricular cardiomyopathy without the presence of significant pulmonary hypertension can also be a frequently observed aetiology. This disease affects over 1.6 million people in the USA1 and around 70 million worldwide5; however, only 0.5% of patients with TR undergo tricuspid valve repair or replacement6.
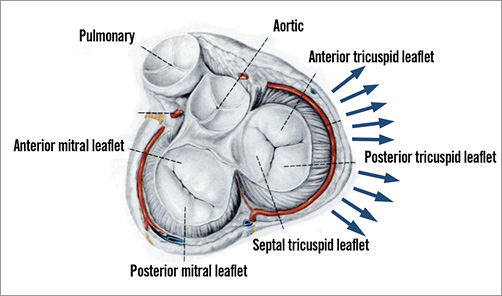
Figure 1. Anatomy of the tricuspid valve.
In its early stages, TR may be well tolerated and managed with optimal medical therapy7. However, the clinical management of this valve disease can be very challenging and it may be affected by several factors leading to left and right heart failure, with consequent worsening of the functional status and quality of life of the patient8. Despite optimisation of medical treatment, many patients still continue to be symptomatic and conservative treatment may not prevent the progression of the disease. Uncorrected TR can be considered self-perpetuating, becoming very incapacitating9. For a long time, functional TR was considered to be purely a consequence of left-sided overload and consequently managed with correction of left-sided valve disease. However, baseline severe TR has now been recognised as a predictor of worse outcome after mitral or aortic valve treatments10-12.
Based on all of these considerations, the new interest in the field of transcatheter interventions for TR is not surprising13-15. As is known for left-sided valves, the primary indication for the treatment of the tricuspid valve remains the classic surgical approach, especially in the context of a combined right- and left-sided valve repair. The current guidelines suggest that, in patients undergoing left-sided valve surgery, concomitant repair of severe TR or moderate TR with tricuspid annulus dilation is indicated16. Similar to the mitral valve, tricuspid valve repair is the preferred treatment17. Moreover, although the short-term outcome of TR surgery can be judged acceptable, the majority of surgical techniques are affected by unsatisfactory long-term results18. The tailored approach to each individual patient and to specific anatomy seems reasonable19.
Undeniably, in high-risk elderly patients for whom surgery has been denied, the era of transcatheter therapies finds its own rationale in its primary effort in offering less invasive percutaneous approaches targeted to the treatment of TR13,14,20.
Tricuspid valve devices
Since there are different aetiologies that may drive TR, several new technologies are available or under development, indirectly reflecting the complexity of this valve and its apparatus19-21. The preliminary experiences with some of these new technologies are already available, with the limitation of a short or midterm follow-up. This review will explore the main technologies available for the percutaneous treatment of TR.
It is easy to understand that a great many of the concepts which have matured over the years in the field of mitral valve treatment can be transferred to the tricuspid valve. However, the anatomical and functional differences between these two valves and respective ventricles should be considered for both patient screening and the procedure, in order to overcome the specific challenges related to tricuspid valve therapies22. Anatomical features such as large tricuspid annular size, non-planar and elliptical annular shape, frailty of the tricuspid annular tissue, angulation in relation to superior and inferior vena cava, the presence of muscular bands and chordae, proximity of the right coronary artery with consequent injury, risk of occlusion of the coronary sinus or vena cava outflow tract, and the presence of pacemaker leads need to be taken into consideration14,23,24.
Similar to mitral valve percutaneous approaches, the treatment of TR includes leaflet and coaptation devices, annuloplasty devices and transcatheter replacement. Moreover, another technology conceived for the “right side” is single or double heterotopic caval valve implantation, known as CAVI (Table 1).
Leaflet devices
THE MITRACLIP® SYSTEM
The off-label use of the MitraClip System (Abbott Vascular, Santa Clara, CA, USA) can be considered the most popular percutaneous approach used for the treatment of TR25-27 (Figure 2A). Indeed, grasping of the tricuspid leaflets has been successfully used for the treatment of TR in selected high-risk patients with malcoaptation of the tricuspid valve28-30. This procedure can be performed via the transjugular access but is to date preferably employed using a transfemoral approach28.
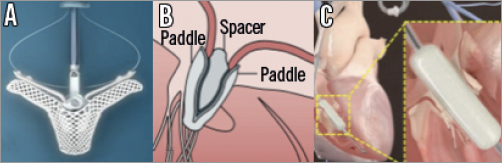
Figure 2. Leaflet and coaptation devices. A) MitraClip System (Abbott Vascular, Santa Clara, CA, USA). B) PASCAL System (Edwards Lifesciences, Irvine, CA, USA). C) FORMA Repair System (Edwards Lifesciences, Irvine, CA, USA).
Although there is extensive experience of this system in the mitral space, several considerations should be taken into account in the case of the tricuspid valve, specifically with regard to the limited steering of the MitraClip guiding catheter in the right atrium, due to the anatomical features of the right atrium and annular plane20. In view of this, the MitraClip System has been re-engineered and will be used in the TRILUMINATE trial (NCT03227757) to study the safety and effectiveness of this tricuspid valve repair system. The first patient was enrolled in the TRILUMINATE trial in August 2017 and recruitment is proceeding worldwide at over 20 sites.
Another important point is the heterogeneous anatomy of the tricuspid valve that provides varied mechanisms for grasping31. Vismara et al32 showed that tricuspid valve edge-to-edge repair can be performed in over 21 positions and combinations, when a single- or double-clip strategy is considered. Moreover, grasping the septal and anterior leaflets allowed the best post-procedural outcome. High-quality transoesophageal images by echo, including 3D and transgrastric views, are mandatory20.
Recently, the early outcome of 64 patients in 10 international centres was reported28. The device success was very high, with a rate of 97% and an incidence of in-hospital mortality of 5%. The anteroseptal commissure was the target of the grasping in 80% of the patients. No cases of device migration were encountered. Orban et al33 also demonstrated the sustained results of the percutaneous edge-to-edge technique on TR at six-month follow-up, with the reduction of TR by at least one grade in 90% of the 50 patients recruited. Different implantation techniques have been developed in order to obtain a satisfactory result. Indeed, a triple orifice tricuspid valve is created when the clips are placed between the septal and anterior leaflets as well as the septal and posterior leaflets, whereas grasping exclusively the septal and the anterior leaflets, which is the most employed technique nowadays32,34, induces a bicuspidalisation of the tricuspid valve.
MitraClip therapy might also be useful for the treatment of degenerative TR, although as yet only single case series have been published and further studies are warranted to confirm the efficacy of this therapy in TR aetiology26,33.
THE PASCAL SYSTEM
The single-centre experience of tricuspid valve repair with the novel PASCAL system (Edwards Lifesciences, Irvine, CA, USA) has recently been described35 (Figure 2B), reporting a good procedural result with residual moderate TR and a reasonable final tricuspid gradient. This system was initially developed for the repair of the mitral valve. In a subsequent series, the PASCAL system, which incorporates a spacer and enables independent leaflet grasping in challenging tricuspid anatomies, was successfully used in eight of nine compassionate use patients with severe TR and large leaflet coaptation gaps. However, further research in terms of device safety and efficacy when compared to other edge-to-edge repair systems is warranted.
Coaptation devices
THE FORMA REPAIR SYSTEM
The FORMA Repair System (Edwards Lifesciences) is a valve spacer that occupies the tricuspid orifice area and therefore acts as a coaptation device by providing a surface for native leaflet coaptation36 (Figure 2C). It has been employed in the case of a tricuspid annular dilatation with functional TR. It consists of a polymer balloon spacer available in different sizes (12 mm, 15 mm and 18 mm) placed in the regurgitant orifice area, attached to a thin nitinol rail fixed to the subclavian vein and the apex of the right ventricle. The larger spacer requires a 24 Fr sheath introducer, and its final size is achieved within the vascular system by passive expansion via eight holes in the spacer shaft. The procedure is performed through a subclavian access or left axillary venous access, which requires a surgical cut-down19-21. The procedure is performed under transoesophageal echo and fluoroscopic guidance. The spacer has two radiopaque markers that assist during positioning and the system is advanced over the rail within the tricuspid annular plane. A right ventriculography can be performed in order to detect the tricuspid annular plane and the anchoring zone in the apex. After positioning, the device is proximally locked at the subclavian region and the residual rail is coiled and placed into a subcutaneous pre-pectoral pocket.
The first-in-man experience of the FORMA device in seven patients was presented in 201537, showing a favourable device implantation, which remained sustained at one-month follow-up. The midterm outcome up to one year in 18 patients treated with the FORMA system in three centres in Canada and Switzerland has recently been reported38. The FORMA system can also be employed in cases of massive or torrential TR, with a magnitude of reduction of TR that is proportional to the severity of the baseline (as shown by Kodali et al at TCT 2017, Denver, CO, USA). In the presentation of the early feasibility study in the USA, these authors also showed that this system proved to be feasible; however, it is associated with infrequent distal dislodgement of the distal anchors, with consequent right ventricle perforation. The next generation of the device is supposed to overcome this issue.
Further information will be derived from the SPACER (The Repair of Tricuspid Valve Regurgitation Using the Edwards TricuSPid TrAnsCatheter REpaiR System [NCT02787408]) trial, a multicentre, international, prospective single-arm feasibility study that will be completed in 2020; the trial is still active but not recruiting.
Annuloplasty devices
Different devices have been designed or adapted from the mitral valve for use in the tricuspid valve in order to treat functional TR caused by annular dilatation percutaneously. Among them are the Cardioband System (Edwards Lifesciences), the Trialign™ Percutaneous Annuloplasty System (Mitralign, Tewksbury, MA, USA), the TriCinch™ System (4Tech Cardio Ltd., Galway, Ireland), the TRAIPTA device (Transatrial intrapericardial tricuspid annuloplasty; National Institutes of Health, Bethesda, MD, USA), the Millipede annular ring (Millipede, LLC, Ann Arbor, MI, USA), and the MIA™ annuloplasty implant (Micro Interventional Devices, Inc., Newtown, PA, USA)19-21. Lastly, another device, which is still experimental in pigs, is the pledget-assisted suture tricuspid annuloplasty to create a double-orifice valve, known as PASTA.
THE CARDIOBAND SYSTEM
The Cardioband is a transcatheter system for direct annuloplasty, which consists of a sutureless surgical-like Dacron band placed on the atrial side of the valve by means of multiple anchors, which allows real-time adjustment of the implant in order to reduce the annular dimension in a tailored way. It has been employed for functional mitral regurgitation with good results39,40 (Figure 3A). This device represents another possible option for patients affected by functional TR; therefore, it has been slightly modified to facilitate tricuspid annular implantation, with the aim of reducing the annular dimensions. The Cardioband transcatheter tricuspid valve reconstruction system recently obtained CE mark approval for use in Europe. The concept and the implant technique are similar to those of mitral valve repair and familiarity with the Cardioband in mitral users could facilitate the learning curve in the treatment of the tricuspid valve41. The safety and efficacy of the Cardioband for a surgical-like undersized direct annuloplasty is under evaluation in the TRI-REPAIR study (TrIcuspid Regurgitation RePAIr With CaRdioband Transcatheter System [NCT02981953]), a single-arm, multicentre (Germany, France, Italy), prospective study. This study includes symptomatic patients affected by chronic functional TR with annular diameters ≥40 mm and sPAP ≤60 mmHg. Preliminary results of the study presented at EuroPCR 2017 (Paris, France) showed, in 20 patients with TR treated with the Cardioband, a 27% reduction of septolateral tricuspid annular dimension with consequent improvement in TR and clinical performance.
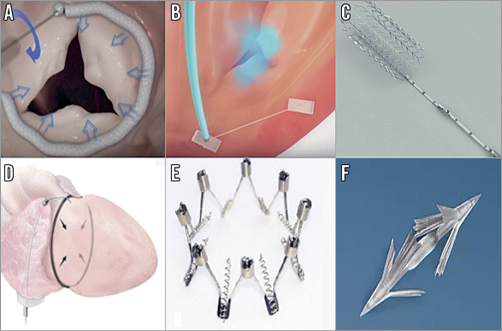
Figure 3. Annuloplasty devices. A) Cardioband System (Edwards Lifesciences, Irvine, CA, USA). B) Trialign Percutaneous Annuloplasty System (Mitralign, Tewksbury, MA, USA). C) TriCinch System (4Tech Cardio Ltd, Galway, Ireland). D) TRAIPTA device (Transatrial intrapericardial tricuspid annuloplasty; National Institutes of Health, Bethesda, MD, USA). E) Millipede annular ring (Millipede, LLC, Ann Arbor, MI, USA). F) MIA annuloplasty implant (Micro Interventional Devices, Inc., Newtown, PA, USA).
THE TRIALIGN DEVICE
Mitralign is a system originally designed for mitral valve repair and then adapted for the tricuspid valve and re-named Trialign42,43 (Figure 3B). This annuloplasty system employs, via a 14 Fr transjugular approach, pledgeted sutures, positioned in the tricuspid annulus and then cinched together with a dedicated plication-locking device. It mimics the surgical Kay procedure, with the aim of reducing TR by a bicuspidalisation technique, obtained with the plication of the anterior and posterior annulus. First-in-man experience was carried out in 2014. Currently, a strategy using two pairs of pledgets might also be feasible, although it is limited to small case series and without evidence of superiority of the two-pair implantation strategy over a single pair one44. A potential issue of this procedure is related to the infrequent pledget dehiscence that may depend on pledget distance45. The safety and feasibility of the Trialign are currently under investigation in the SCOUT study (Early Feasibility of the Mitralign Percutaneous Tricuspid Valve Annuloplasty System [NCT02574650]) that will be completed in 2019. The preliminary 30-day results of the SCOUT study confirmed the safety of the Trialign system, which reduced the tricuspid annulus and improved quality of life46.
THE TRICINCH SYSTEM
This device is composed of a corkscrew anchor that will be anchored in the anteroposterior annulus and then retracted into the inferior vena cava by a Dacron band that connects the anchor to a self-expanding nitinol stent (Figure 3C). The stent is available in different sizes (27, 32, 37 and 43 mm) in order to ensure adequate oversizing in the vena cava, in correspondence to the hepatic junction. The tension is maintained through a wire that joins the screw to the stent. The first-in-human experience was reported in an old patient with severe functional TR, through a conventional femoral vein access, with reduction of TR from severe to moderate-to-severe; the authors also described a modest improvement of the symptoms47-49. The PREVENT trial (Percutaneous Treatment of Tricuspid Valve Regurgitation with the TriCinch System [NCT02098200]) was completed in October 2017. This trial showed an implant success rate of 75% (18/24). During the twelve-month follow-up, four (17%) late anchor detachments were observed. A new generation of the device is currently under preclinical evaluation with the intention of overcoming these issues (Denti P. Preclinical experience with new percutaneous tricuspid valve repair system. Presented at EuroPCR 2017, Paris, France). Other information regarding the outcome of this device will be forthcoming from the PROTECT feasibility study (Percutaneous Treatment of Tricuspid Valve Regurgitation With the TriCinch System [NCT03294200]) that will be completed in 2020.
THE TRAIPTA DEVICE
This system is an experimental epicardial nitinol loop, placed around the atrioventricular groove providing an external annuloplasty50 (Figure 3D). The device is positioned in the epicardial space through a puncture of the right atrial appendage via a transfemoral access. The puncture in the right atrium will finally be closed with an occluder device. It has been employed in experimental models in 16 swine in which the device was delivered successfully. Nine animals were ultimately used in a survival experiment: they underwent a post-procedural evaluation that demonstrated a reduction of the tricuspid annular area by 59%, with a 49% reduction of the septal-lateral diameter and a 31% reduction of the anteroposterior diameter. Some concerns about translating this procedure to humans include the increased risk of cardiac tamponade due to higher pressure in the right atrium in humans than in animals or the impediment of a safe epicardial access because of adherences created by previous surgical interventions. Presently, no human trials of the TRAIPTA have been registered, although a new TRAIPTA device for human testing is currently under development.
THE MILLIPEDE ANNULAR RING
This technology is a device composed of expandable and predefined size contractible rings that create a zigzag frame. It has multiple thin anchors to fix it on the atrial side of the tricuspid annulus that mimic traditional surgical annuloplasty (Figure 3E). This device is still under investigation. It was previously used for mitral valve repair and showed encouraging first results in humans20.
THE MIA SYSTEM
The MIA is a device composed of ultra-low mass proprietary compliant PolyCor™ anchors and MyoLast™ implantable elastomer (both Micro Interventional Devices, Inc.) that allows bicuspidalisation of the tricuspid valve, with consequent reduction of the tricuspid area21 (Figure 3F). This innovative and attractive device is still under study in the STTAR trial (Study of Transcatheter Tricuspid Annular Repair). Four patients enrolled in the trial have been treated with success.
Caval valve implantation (CAVI)
Off-label caval implantations of aortic valves have been used as a last-resort approach for the treatment of TR with refractory right heart failure. The implantation of this valve in the caval space allows reducing the negative effects of the venous congestion and consequently may lower the symptoms of heart failure, although it does not directly act upon the severity of the TR itself. Apart from the Edwards SAPIEN prosthesis (Figure 4A), a dedicated bicaval valve system known as TricValve® (P&F Products and Features GmbH, Vienna, Austria) has been developed51-55 (Figure 4B). This valve consists of two separate trileaflet pericardial tissue valves, which are mounted on self-expanding nitinol stents. Two bioprosthetic valves are available, with sizes ranging from 28 to 43 mm (up to 38 mm for the superior vena cava and 43 mm for the inferior one). Both valves are delivered through a transfemoral approach in a dedicated 27 Fr catheter. The inferior vena cava device is deployed with the jacketed portion into the right atrium and a waist in the hiatus of the diaphragm to provide drainage of the hepatic venous system. The valve stent for the superior vena cava is funnel-shaped to adapt to the junction between the vena cava and right atrium. Preclinical studies in animal models (sheep) have demonstrated promising haemodynamic results of this technique.
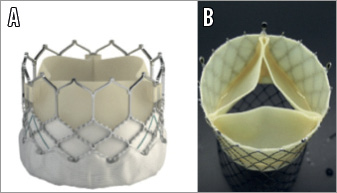
Figure 4. Caval valve implantation (CAVI). A) Edwards SAPIEN prosthesis (Edwards Lifesciences, Irvine, CA, USA). B) TricValve (P&F Products and Features GmbH, Vienna, Austria).
The first-in-human implantation of a self-expanding valve in the inferior vena cava was described in 201151. Results from a multicentre registry including 25 patients have recently been published56. Nineteen patients were treated with single valve implantation, while six had bicaval implantation, with the use of both balloon-expandable and self-expanding valves. Procedural success was achieved in 96%. Thirty-day and in-hospital mortality was 8% and 16%, respectively. Just one case of valve migration requiring surgery was reported. Further information about CAVI will be delivered by the HOVER single-centre registry (Heterotopic Implantation of the Edwards-SAPIEN XT Transcatheter Valve in the Inferior Vena Cava for the Treatment of Severe Tricuspid Regurgitations [NCT02339974]) and by the TRICAVAL single-centre randomised trial (Treatment of Severe Secondary TRIcuspid Regurgitation in Patients With Advance Heart Failure With CAval Vein Implantation of the Edwards Sapien XT VALve [NCT02387697]).
Tricuspid valve replacement
Orthotopic tricuspid valve replacement has been employed in case of degenerated surgical bioprostheses or failure of surgical rings with usage of the balloon-expandable Edwards SAPIEN (Edwards Lifesciences) (Figure 4A) or the Melody® valve (Medtronic, Minneapolis, MN, USA) (Figure 5A)20,57,58. To date, the GATE™ self-expanding valved stent (NaviGate Cardiac Structures Inc., Lake Forest, CA, USA) (Figure 5B) is the only available device that has been used to perform transcatheter tricuspid valve replacement in humans for compassionate use (presented at EuroPCR 2017, Paris, France) and whose results in preclinical models have recently been published. The NaviGate valve is a biological valved stent that comprises a specially configured nitinol alloy stent into which is mounted a trileaflet valvular mechanism produced from equine pericardium.
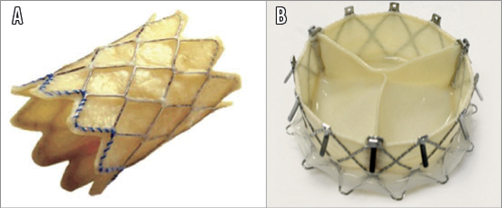
Figure 5. Tricuspid valve replacement. A) Melody valve (Medtronic, Minneapolis, MN, USA). B) GATE self-expanding valved stent (NaviGate Cardiac Structures Inc., Lake Forest, CA, USA).
Conclusions
The preliminary results of transcatheter techniques for the treatment of TR have shown feasibility and safety, with an encouraging reduction in TR grade and improvement of New York Heart Association class and quality of life. The screening process, patient selection and anatomical definition as well as the choice of the “right device for the right patient” still remain to be defined, supported by evidence to be provided by the upcoming studies and trials. Moreover, a correct definition of endpoints of success and failure for tricuspid valve procedures - as previously carried out with the aortic and mitral valve, respectively, with the VARC-2 and MVARC criteria - is warranted, in order to evaluate the results of percutaneous TR techniques better20.
Funding
The Medizinische Klinik und Poliklinik I der Ludwig-Maximilians Universität München receives an institutional research grant from Abbott Vascular.
Conflict of interest statement
C. Grasso is a proctor physician for Abbott Vascular. D. Braun has received speaker honoraria from Abbott Vascular. J. Hausleiter has received speaker honoraria from Abbott Vascular and Edwards Lifesciences. G. Nickenig has received honoraria for lectures or advisory boards, participation in clinical trials, research funding from Abbott, Boston Scientific, Edwards, Medtronic and Mitratech. A. Popolo Rubbio has no conflicts of interest to declare.

