Considering the incidence of tricuspid regurgitation (TR) and its implications on functional status and long-term survival, TR is currently undertreated. With the increasing adoption of catheter-based treatments of other types of structural heart disease there is a growing interest and need for effective interventional treatment also for tricuspid regurgitation. Therefore, multiple treatment approaches are under investigation; however, in this chapter we focus exclusively upon tricuspid replacement.
Transcatheter replacement of the tricuspid valve
Due to the high operative mortality of isolated tricuspid surgery, it is probably correct to predict that the percutaneous approach will be the treatment of choice in patients requiring tricuspid surgery. Considering the large unmet need for an interventional option for inoperable patients with tricuspid regurgitation, percutaneous valve implantation could be a feasible alternative, besides interventional valve repair. From the interventional perspective, there are two basic approaches, depending on the anatomic site of prosthetic valve implantation – an orthotopic versus a heterotopic valve replacement.
In orthotopic valve replacement, the prosthetic valve is deployed at the level of the TV annulus between the right ventricle (RV) and right atrium (RA). This approach was investigated by Boudjemline et al by means of implanting a double-disc nitinol stent with a semilunar valve into the tricuspid annulus. Due to the anatomic structure and the flexibility of the surrounding myocardium, this site of implantation offers little resistance for orthotopic long-term fixation of stent-based valves. In functional TR, annulus diameter may reach >50 mm associated with a loss of anatomic landmarks between the RV and RA. A device intended for orthotopic TV replacement would therefore require unique solutions for stent-valve fixation as well as tissue valve engineering (e.g., a 50 mm tissue valve would require a profile height of >30 mm). To our knowledge only one company is presently working on such a demanding system for orthotopic valve replacement (TRicares GmbH, Aschheim, Germany).
So far in patients, orthotopic tricuspid valve replacement has been used with promising results only as a valve-in-valve or valve-in-ring procedure using either the balloon-expandable Edwards SAPIEN (Edwards Lifesciences, Irvine, CA, USA) or the Melody® (Medtronic, Minneapolis, MN, USA) valve. As with most low-volume procedures, there are no published data from larger patient series regarding the outcome and long-term function of these devices in the tricuspid position.
A feasible alternative to orthotopic approaches is caval valve implantation (CAVI), which involves the implantation of stent valves in a heterotopic positon into the inferior vena cava (IVC) and superior vena cava (SVC). This concept has been investigated preclinically with encouraging results and has been used on compassionate grounds in humans. However, pulsatile systolic blood flow reversal in the caval veins is a prerequisite for the proper function of the caval valves. This is reflected by a profound V-wave atrial flow pattern which is required prior to heterotopic implantation.
Heterotopic valve design
The CAVI procedure is technically simple and can be performed rapidly without interference to cardiac structures. Devices do not interfere with any pre-existing transtricuspid pacemaker or defibrillator leads, which might represent a limitation for orthotopic procedures on the tricuspid valve. Under the condition of severe chronic TR, there is a considerable variation of the anatomic diameter of the SVC and IVC, which frequently exceeds the suitable range for implantation of current, commercially available devices. The SVC might dilate up to 40 mm in diameter and the IVC up to 35 mm. With the Edwards SAPIEN XT valve, these challenges have been partially compensated for by pre-stenting the caval veins prior to valve deployment for downsizing and improved valve anchoring by Laule et al who showed haemodynamic improvement even with a single valve only in the IVC.
In the caval position, self-expandable devices specifically designed for caval valve implantation with little radial force are likely to be a superior alternative as they do not require pre-stenting of the landing zone. Self-expandable valves for commercial use are currently under development by the company P & F Products & Features Vertriebs GmbH, Vienna, Austria. The anatomic variation is addressed for the SVC by a device with a one-size valve diameter of 30 mm in the tubular part of the stent, but with a variable hip protrusion of up to 45 mm. The IVC device is deployed with the jacketed valve part in the right atrium and a waist in the hiatus of the diaphragm, allowing hepatic veins to drain unobstructed into the IVC (Figures 1 and 2).
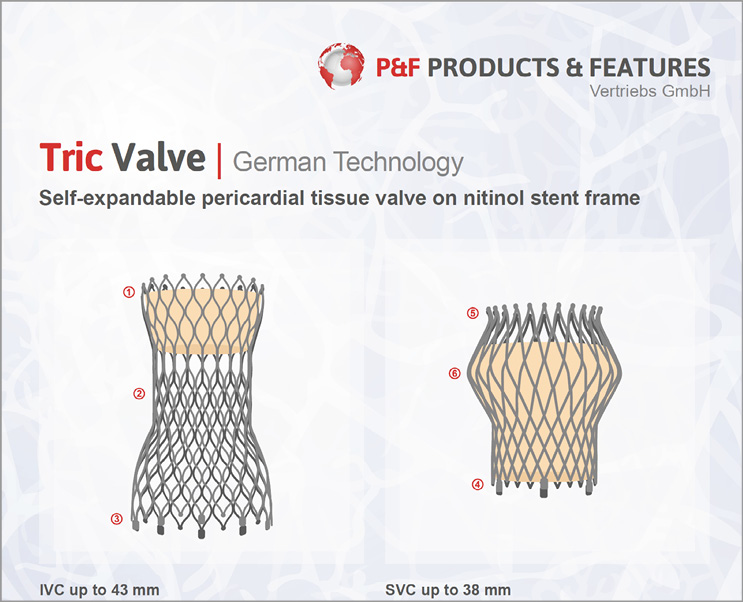
Figure 1. The TricValve (produced by P & F Products & Features Vertriebs GmbH, Weßling, Germany), under development.
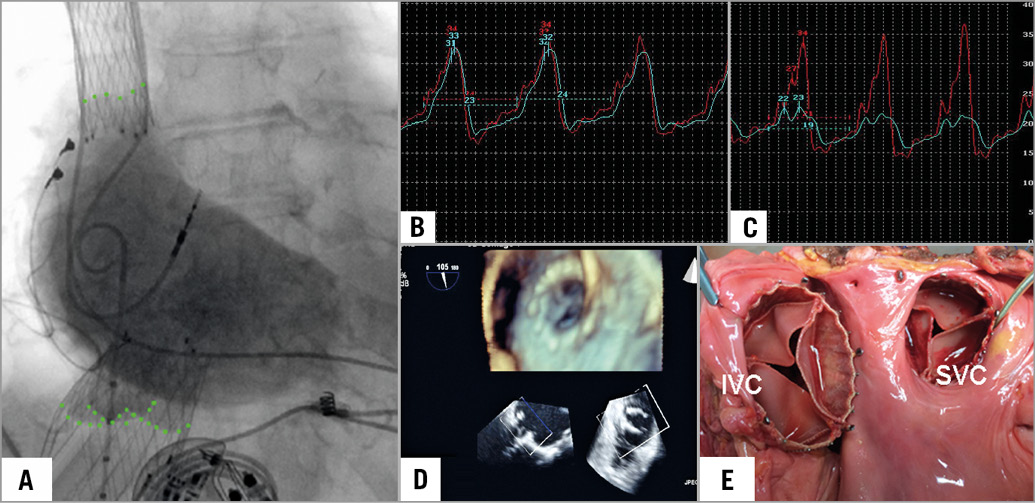
Figure 2. Caval valve implantation. A) Position of self-expanding valves in the SVC and the IVC. Note the valve leaflets marked by angiography. B) & C) Pressure measurement confirms a reduction of V-wave and mean pressure in the inferior vena cava from 32 mmHg to 23 mmHg and 24 mmHg to 19 mmHg, respectively. D) Device function is observed in transoesophageal echocardiography. E) Macroscopic specimen demonstrating the device position in the SVC and IVC. (image copyright European Heart Journal)
Haemodynamics and anatomic consequences following bicaval valve implantation (CAVI)
Following implantation of the TricValves, the valves resemble cardiac valves (Moving image 1). Caval V-waves are flattened, while atrial V-waves increase following the reduced capacity volume for the regurgitant blood flow. In the acute phase, mean caval pressures are only slightly reduced (2-3 mmHg). In the following weeks, the remodelling of the RV due to the reduced volume load initiates a decrease of RA and caval vein pressures. Post-mortem examinations showed an ingrowth of the stent frame, while a thrombus on the valve was never seen. However, all patients were on oral anticoagulation.
The persistence of right atrial volume overload, the ventricularisation of the RA and the increase of RV afterload are potential limitations of the procedure. Its long-term impact on RA and RV function is currently unknown. Post-implant device function has been observed up to 24 months following implantation in one patient, resulting in a profound clinical improvement, from NYHA Class IV to II and a normalisation of impaired liver function.
Conclusion
CAVI with the TricValve is a relatively simple procedure. However, valve design has to cover a great range of caval vein anatomy. The haemodynamic concept is convincing and allows the RV to recover. Clinical experience is presently restricted to compassionate cases; commercialisation is on its way.
Conflict of interest statement
H.R. Figulla, K. Kiss, and A. Lauten receive consultancy fees from P&F Products & Features, Vienna, Austria.
Supplementary data
Moving image 1. An echo view of the IVC with a TricValve. Note the movement of the leaflets, resembling a cardiac valve. Colour code demonstrates no regurgitant flow.
Supplementary data
To read the full content of this article, please download the PDF.
An echo view of the IVC with a TricValve. Note the movement of the leaflets, resembling a cardiac valve. Colour code demonstrates no regurgitant flow.

