Introduction
Functional tricuspid regurgitation (FTR) is a frequent finding in elderly patients, due to annular dilatation and leaflet tethering, as a consequence of right ventricular remodelling. It is associated with right heart failure, reduced quality of life and poor outcome. The standard treatment for FTR is surgical repair. However, many patients are not surgical candidates, due to severe right ventricular failure or associated comorbidities. In patients deemed to have a prohibitive surgical risk, the need of alternative percutaneous options is evident. Novel transcatheter therapies are emerging for the treatment of significant tricuspid regurgitation (TR). TriCinch™ (4Tech Cardio Ltd, Galway, Ireland) is a percutaneous device designed for tricuspid valve (TV) remodelling, to reduce the annular dilatation and prevent further right heart decompensation. The first-in-human feasibility trial is currently ongoing and, as experience expands, procedural refinements and optimised patient selection will arrive.
Device description
Name and manufacturer: The TriCinch system (4Tech Cardio Ltd, Galway, Ireland).
Approval status: First-in-human multicentre European study ongoing. CE mark expected in 2017.
Implant technology: The TriCinch system comprises two components: 1) a stainless steel corkscrew implant, to be placed in the anterior annulus of the TV, in proximity to the anteroposterior commissure (APC); 2) a self-expanding nitinol stent that is deployed below the hepatic region of the inferior vena cava (IVC) (Figure 1A).
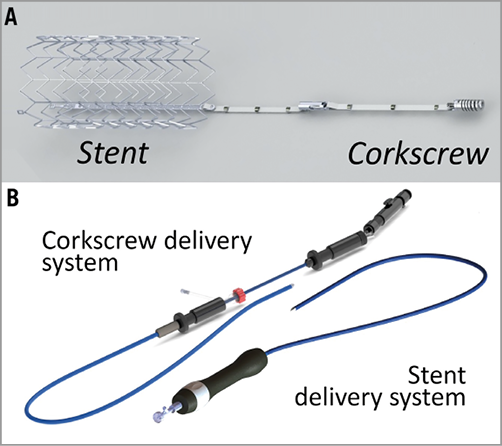
Figure 1. The TriCinch system. The TriCinch system is composed of the implant (A), featuring a self-expanding inferior vena cava stent (left), an annular corkscrew (right), and two delivery systems (B) for corkscrew (left) and stent (right) placement.
Delivery system: The transfemoral delivery system consists of two components: 1) a delivery system that enables transcatheter placement of the corkscrew implant in a supra-annular TV position; 2) a stent delivery system for stent deployment in the IVC (Figure 1B).
Delivery method: Transfemoral venous access.
Implant sizes: Stent implants are available in four different sizes (27, 32, 37 and 43 mm) in order to allow oversizing of 30% also in dilated IVC (18 to 35 mm).
Procedural details
The procedure is stepwise and reproducible, with expected implantation time under one hour, and is reversible at every step, to enhance control and safety (Figure 1, Moving image 1).
1) Access: The TriCinch catheter is inserted over the femoral vein to the IVC, through a groin puncture, and then steered towards the right atrium (Figure 2A).
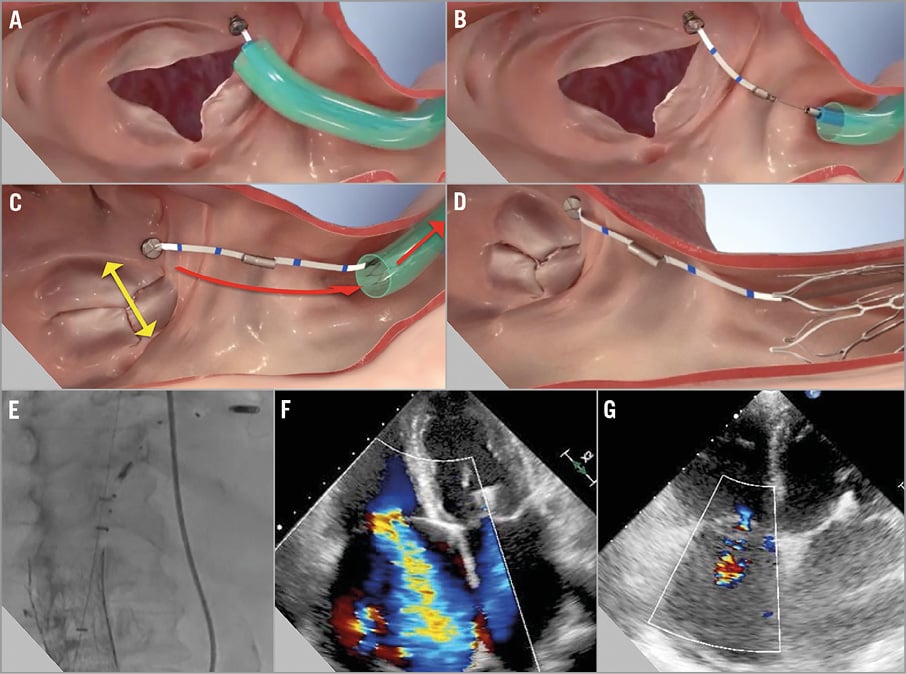
Figure 2. The TriCinch procedure. The procedure begins with access to the left atrium targeting the tricuspid annulus (A), then corkscrew implant deployment (B), system tensioning to remodel the tricuspid valve (C), and tension stabilisation with stent implantation in the inferior vena cava (D). Periprocedural fluoroscopic implantation guidance and confirmation of device targeting (E). Annular dilatation and tricuspid regurgitation reduction: severe (4+) at the preoperative TTE (F) to moderate (2+) at six-month follow-up (G).
2) Corkscrew implant deployment: Once the tricuspid valve is accessed, the corkscrew is anchored on the TV annulus, in a region close to the APC (Figure 2B).
3) Stent implant deployment: After the first delivery system is retrieved, the stent is advanced and coupled to the corkscrew.
4) Tricuspid remodelling: The whole system is then tensioned to remodel the TV and reduce annular dimension and TR (Figure 2C). The tension is maintained via deployment of the stent in the IVC (Figure 2D).
These steps are performed under live transoesophageal and intracardiac echocardiography, as well as fluoroscopic guidance. At the end of the procedure, the delivery system and the venous introducer are removed.
Targeted population
The TriCinch therapy is designed to offer a less invasive, transcatheter option for treating significant TR in patients deemed to have prohibitive surgical risk.
The targeted population includes:
– Patients with isolated severe functional TR due to annular dilatation (>40 mm at echocardiography), symptomatic despite maximal medical therapy, who are deemed to be inoperable or at high risk for open heart surgery.
– Patients undergoing transcatheter mitral valve treatment with concomitant severe functional TR or with less than severe TR in the presence of annular dilatation.
The safety of this therapy will be demonstrated in clinical trials, but comorbidities, biventricular dysfunction, and pulmonary vascular disease will still influence the outcome in these patients.
We report a case example performed at our institution in January 2016.
A 77-year-old female patient presented in May 2015 with significant FTR (severe TR 4+, hepatic vein flow) (Figure 2F) and severe mitral regurgitation (MR) (degenerative MR, P2 prolapse, LVEF 44%), NYHA Class III and several episodes of right heart failure. Due to comorbidities of severe COPD, chronic renal failure and persistent atrial fibrillation, the patient was deemed a high-risk patient for open surgery. Two MitraClips were implanted resulting in a minimal residual MR. No percutaneous tricuspid therapy was available in our centre at that time. Following a further episode of right heart failure requiring hospitalisation for recompensation four months after, the percutaneous TV repair was planned for the beginning of 2016.
The TriCinch procedure was performed under transoesophageal and intracardiac echocardiography as well as fluoroscopic guidance. The delivery system was advanced from the right femoral vein and steered to the anterior tricuspid annulus. The corkscrew was implanted into the annulus at the target area near the APC, as planned in a preoperative CT scan. Then the stent, and the whole system, was tensioned to remodel and reduce TV dimension, until optimal outcome was observed at echocardiography. Tension was maintained via deployment of a 32 mm self-expanding stent in the IVC (Figure 2E). Post-procedural echocardiography showed reduced TR (moderate to severe, 3+) and 50% reduction in the effective orifice area (EOA pre 2.03 cm2 to post 1.07 cm2).
At six-month follow-up, the tension had been maintained over the TV, with sustained improvements at echocardiography (TR 2+) (Figure 2G) and clinical status (six-minute walk test from 150 m at baseline to 250 m) with corresponding higher quality of life and no further episodes of heart failure requiring hospitalisation. This case shows the feasibility of percutaneous tricuspid valve treatment, suggesting encouraging quality of life improvement.
Percutaneous mitral treatment led to improvement in MR, but baseline moderate to severe TR is a strong predictor of survival, heart failure hospitalisations and quality of life. Treating TR simultaneously with the MV percutaneous procedures could stabilise the results by preventing right heart decompensation, aiming at surgical standards.
Ongoing studies
Following complete preclinical validation, the first-in-human multicentre clinical study is evaluating the feasibility, safety and performance of the TriCinch transcatheter tricuspid remodelling system in patients with FTR. The PREVENT clinical study (Percutaneous Treatment of Tricuspid Valve Regurgitation With the TriCinch System; ClinicalTrials.gov Identifier: NCT02098200) is currently ongoing and enrolling in Europe.
Unique features
The TriCinch system features a unique annular implant, designed to reduce large tricuspid annulus dimensions by targeting the optimal remodelling area on the TV, and to respect the right ventricular morphology with its supra-annular implantation.
The corkscrew supra-annular fixation, as in surgery, and the stepwise procedure, which is reversible, until two thirds of the stent deployment, allow a simple and predictable percutaneous procedure, and guarantee an enhanced level of safety throughout the procedure.
The procedure is performed on the beating heart, allowing the tailoring of the remodelling to the patient’s specific valvular anatomical geometry, in fully physiological conditions under direct echocardiographic control.
The TriCinch can be used in combination with other valvular therapies, and it preserves the native anatomy and the subvalvular structures, allowing future options.
Potential improvements
Further refinement in the targeted patient population will validate and enhance the clinical response to this therapy. Fusion imaging may improve procedural guidance, which remains an issue in some patients with a challenging echocardiographic window.
Conflict of interest statement
F. Maisano is a co-founder of 4Tech. B. Rosser and M. Taramasso have no conflicts of interest to declare.
Supplementary data
Moving image 1. Transcatheter interventions for tricuspid regurgitation: implantation of TriCinch (4Tech).
Supplementary data
To read the full content of this article, please download the PDF.
Transcatheter interventions for tricuspid regurgitation: implantation of TriCinch (4Tech).

