Device description
The Trialign device (Mitralign, Inc., Boston, MA, USA) consists of three main components: an 8 Fr articulating catheter for wire delivery, a pledget catheter that is preloaded with both the ventricular and the atrial part of the pledget (which have a size of 4×8 mm), and a plication lock device, which is used to perform the cinching and to lock the sutures, to keep the pledgets in position (Moving image 1).
Procedural details
Under general anaesthesia, transjugular access is obtained by placing two 14 Fr sheaths. The right coronary artery is accessed via the femoral or radial artery with a 6 Fr guiding catheter and a soft PTCA wire is introduced into the artery as a fluoroscopic marker. The Trialign guiding catheter is loaded with the deflectable wire delivery catheter, which is advanced across the tricuspid valve and positioned into the right ventricle under fluoroscopy. With two- and three-dimensional (2D and 3D) transoesophageal echocardiographic (TEE) guidance the catheter is then articulated under the annulus to approach the posteroseptal commissure. For this purpose mid-oesophageal and deep gastric echo views are most helpful. An insulated radiofrequency wire is advanced and positioned 2-5 mm from the base of the leaflet (hinge points) within the annulus, in order to have enough tissue for a stable pledget position. The wire is directed towards the right atrium to avoid interference with the right coronary artery, which is confirmed by TEE as well as fluoroscopy. The wire is then snared from the jugular vein using the second sheath and externalised. The pledget delivery catheter is advanced over this wire to cross the annulus to the right ventricle. The wire is removed and the ventricular part of the pledget is delivered and placed in the subannular region of the ventricle. Upon withdrawal of the pledget delivery catheter, the remaining half of the pledget is extruded and cinched on the atrial surface of the annulus. The pledget delivery catheter is withdrawn and the suture, which is connected to the pledget, is placed outside the patient.
The guide catheter loaded with the wire delivery catheter is then advanced again into the right ventricle, curved and steered towards the anteroposterior commissure under TEE guidance. Care has to be taken to identify a puncture site which is 24 to 28 mm away from the first pledget. In case of too small a distance the cinching might not lead to a sufficient reduction of the annulus diameter. On the other hand, if the distance is too great the tension on the pledgets may cause a pull through the annulus. The measurement of the distance between the two puncture sites is performed after wire crossing and repeated after pledget delivery. In case of an incorrect puncture site the wire crossing can be repeated at a different position.
After placement of the second pledget, a dedicated plication lock device is advanced over the two sutures to bring the two pledgets close to each other, thereby plicating the annulus, which in most cases results in a complete folding of the posterior leaflet creating a bicuspid valve. The degree of cinching is controlled under TEE and can be adapted according to the echocardiographic findings.
Clinical data
The first-in-man procedure was performed at our institution in September 2014.
The 89-year-old female patient suffered from right heart failure due to severe TR with tethered, non-thickened leaflets, severe dilatation of the right atrium and ventricle with slightly impaired right ventricular function. 3D TEE at baseline revealed a tricuspid valve annular area of 14.1 cm2, and an effective regurgitant orifice area (EROA) of 1.35 cm2. After cinching, there was a significant reduction in annular area by 57% to 6.05 cm2 and in EROA by 53% to 0.63 cm2 (Figure 1). Right atrial pressure decreased from 22 mmHg at baseline to 9 mmHg, and LV stroke volume increased from 42 cc at baseline to 72 cc. The patient tolerated the procedure well, was extubated the same day, and discharged on day five following the procedure with significant improvement in effort tolerance.
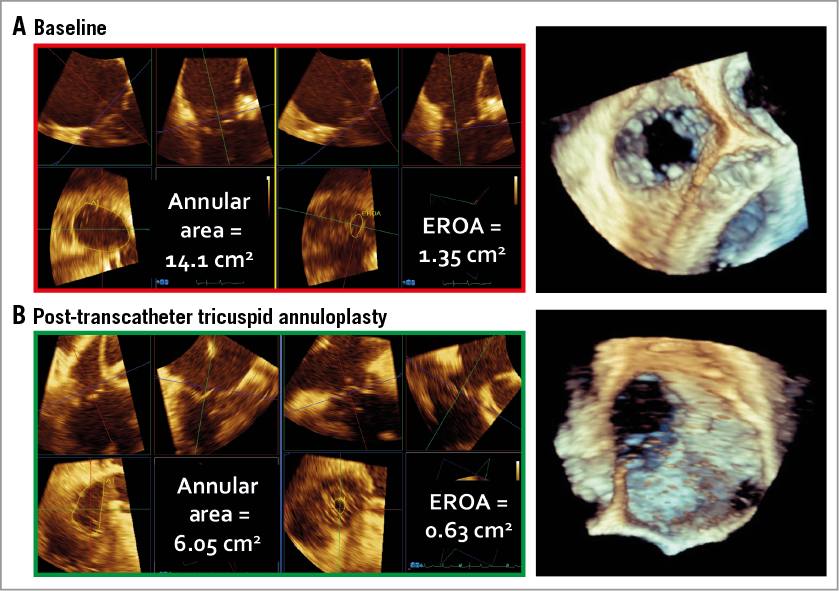
Figure 1. Annular area and effective regurgitant orifice area in 3D transoesophageal echo at baseline (A) and after cinching (B).
Meanwhile, 16 patients have been treated in different centres in Europe and the USA with a good safety profile. There was no procedural death, no myocardial infarction, no stroke or TIA, no acute kidney injury or conduction disturbance, no perforation, and only one bleeding complication at the venous puncture site with no consequences.
Ongoing studies
A feasibility trial (SCOUT) was started in the USA in November 2015 and a CE-mark trial in Europe may start in the third quarter of 2016.
Conflict of interest statement
The author has no conflicts of interest to declare.
Supplementary data
Moving image 1. Animation of procedural steps.
Supplementary data
To read the full content of this article, please download the PDF.
Animation of procedural steps.

