
Abstract
Aims: The aim of this study was to evaluate the safety, technical feasibility and performance of a new transcatheter tricuspid repair system.
Methods and results: Thirty-one adult swine underwent implantation of a transcatheter tricuspid remodelling system under general anaesthesia. The steerable transcatheter device was introduced through a 24 Fr femoral sheath into the right femoral vein and delivered to the tricuspid annulus on the beating heart. A fixation element was implanted into the tricuspid annulus. Following implantation, a second delivery system was used to couple the fixation element with a self-expanding nitinol stent. The device was tensioned to reshape the tricuspid valve and increase the coaptation length of the valve leaflets under echo guidance. Finally, the stent was deployed in the inferior vena cava (IVC) to maintain the tension applied. The transcatheter device was successfully implanted in all animals (n=31). Doppler echocardiography prior to sacrifice showed that tricuspid valve function was stable and normal tricuspid leaflet motion was observed. Cinching of the tricuspid annulus resulted in an increase of leaflet coaptation length of 70% (4.5±0.7 mm to 7.78±1.3 mm), an increase in trans-tricuspid peak velocity of 79% (0.38±0.1 m/s to 0.68±0.1 m/s), and a reduction in septolateral tricuspid valve dimension of 30% (35.2±5 mm to 24.8±5 mm). At necropsy, the fixation element was firmly attached to the annulus within a fibrotic tissue, with no coronary lesions observed and no abnormality visible. The stent was fully deployed in the IVC, without displacement or change in the stent shape.
Conclusions: Percutaneous beating heart remodelling of the tricuspid annulus with a cinching device is safe and feasible.
Abbreviations
APC: anteroposterior commissure
CL: coaptation length
FTR: functional tricuspid regurgitation
ICE: intracardiac echocardiography
IVC: inferior vena cava
MV: mitral valve
RCA: right coronary artery
SL: septolateral
TV: tricuspid valve
Introduction
Functional tricuspid regurgitation (FTR) secondary to left-sided heart disease in the absence of organic lesions of the tricuspid valve (TV) is the most frequent aetiology of TV disease in western countries1. The prevalence of TR in patients with mitral valve (MV) disease is high1,2, and significant TR occurring late after left-heart surgery is observed in up to 40% of patients, with reduced survival1-4.
Late recurrence of TR after left-side surgery is a relevant clinical problem: several studies report a higher long-term mortality rate, lower quality of life, and reduced exercise capacity in patients who developed severe TR after mitral surgery1,3,5.
Redo and TV surgery is associated with high early mortality, ranging from 10% to 37%6-8, and therefore clinicians are often reluctant to recommend redo TV surgery9. Currently, moderate to severe tricuspid TR affects about 1.6 million patients in the USA, of whom only 8,000 undergo tricuspid surgery annually10, resulting in a tremendous number of untreated patients with TR. Severe TR is an independent predictor of long-term mortality (65% one-year survival rate in patients with severe TR compared with 90% in patients without TR)11.
Percutaneous procedures may be attractive alternatives to surgery for patients deemed to be high-risk surgical candidates. Whereas over the past few years the development and clinical use of percutaneous approaches to the aortic and mitral valve have been widespread, only minimal data are available about the feasibility and efficacy of the percutaneous TV approach12,13.
The TriCinch™ system (4Tech Cardio Ltd, Galway, Ireland) is intended for the percutaneous beating heart treatment of the FTR by the remodelling of the TV under echocardiographic and fluoroscopic guidance.
This submission describes the initial preclinical experience with the TriCinch system. The purpose is to evaluate the feasibility, safety and efficacy of the TriCinch system implanted on the native TV using a minimally invasive percutaneous approach.
Methods
The animal studies were conducted in two facilities, the Biotechnology Research Center for Cardiothoracic Applications of Rivolta d’Adda, Italy (CRABCC), and the hybrid animal lab of the University of Zurich, Switzerland. The experiments were performed in accordance with policies and principles of good laboratory practice for animal care and with institutional guidelines, in compliance with Italian Ministry of Health national law (116/92), European Union guideline (86/609/EEC) and Swiss Federal animal protection law and ordinance under the licence 138/2010 issued by Zürich Cantonal Veterinary Office.
Thirty-one pigs, weighing 81-93 kg, underwent the TriCinch procedure. All study animals were anaesthetised and intubated prior to the start of the experimental procedure. Two study groups were considered: the acute study group (n=17), implanted by a completely percutaneous approach, in which we aimed to evaluate the technical feasibility and efficacy of the device, and the chronic study group (n=14), implanted by a hybrid approach, in which we aimed to evaluate safety up to 90 days after implantation.
After loss of postural reflexes following premedication with ketamine (20 mg/kg), azaperone (1.5 mg/kg) and atropine (0.75 mg), the anaesthesia was deepened by a bolus injection of propofol (1-2 mg/kg BW) and the animals were intubated. Anaesthesia was then maintained with 2-3% isofluorane and propofol (2-5 mg/kg/hr). Amiodarone (2-3 mg/kg bolus iv) was administered to stabilise the heart rhythm. Pain management included fentanyl constant rate infusion (0.02 mg/kg/hr) for the duration of the procedure.
THE TRICINCH SYSTEM
The TriCinch system is a percutaneous catheter-based device for the percutaneous treatment of tricuspid regurgitation. The TriCinch system is made up of the following components:
1) TriCinch implant (Figure 1).
The TriCinch implant consists of a fixation element (corkscrew, stainless steel) and of a stabilisation element (stent, nitinol) integrated with a Dacron fabric component.
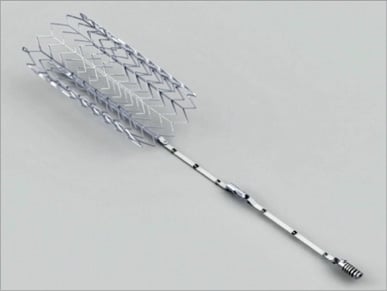
Figure 1. The TriCinch implant consists of a fixation element (corkscrew, StSt) and of a stabilisation element (stent, NiTi) integrated with a Dacron fabric component.
The corkscrew is designed to be implanted in close proximity to the anteroposterior commissure of the TV. The corkscrew is positioned at the desired location by the corkscrew delivery system. The corkscrew is screwed into the tissue of the annulus through rotation of a handle. An integrated radiopaque depth marker within the internal bore of the corkscrew provides visual confirmation that the corkscrew is inserted into the tissue to the correct depth. The overall length of the corkscrew is 14 mm, and it is inserted to a depth of 6.5 mm into tissue. The diameter of the corkscrew is 4.0 mm. Selective right coronary artery angiography is performed to exclude coronary damage after the implant.
The self-expanding stent ensures that the tension applied to the TV is maintained, after deployment of the stent in the inferior vena cava (IVC). The flared design coupled with controlled stent oversizing ensures complete fixation of the stent to the vessel, preventing potential stent displacement in a dilated IVC. The stent used in this preclinical study was 27 mm in diameter and 60 mm in length.
A Dacron band with locking mechanism provides the permanent lock between the corkscrew and the stent. This comprises 50 mm of Dacron attached to the corkscrew, 35 mm attached to the stent, and the 15 mm length of the locking mechanism used to connect these parts of the Dacron band.
TRICINCH DELIVERY SYSTEMS (Figure 2)
The TriCinch system is implanted through the use of two delivery systems, the corkscrew delivery system and the stent delivery system. The corkscrew delivery system is a steerable multifunctional system, which allows the delivery and the fixation of the corkscrew into the TV annular tissue at the target position. The stent delivery system enables the locking of the corkscrew to the stent and the subsequent deployment of the stent in the IVC, and maintains the tension that has been applied within the system.

Figure 2. TriCinch delivery systems.
Implant procedure
FULL PERCUTANEOUS APPROACH (acute group=17 animals)
A 10 Fr sheath was placed in the left femoral vein to permit the use of intracardiac echocardiography (ICE). The steerable transcatheter device was introduced through a 24 Fr femoral sheath in the right femoral vein and delivered to the tricuspid annulus on the beating heart. The distal shroud of the steerable catheter was advanced to the annulus between the anteroposterior commissure (APC) and a location midway along the anterior leaflet, using echo and fluoroscopic guidance. The corkscrew, loaded in the corkscrew delivery system, was implanted in the proximity of the tricuspid valve annulus between the APC and the mid part of the anterior leaflet. Right coronary artery (RCA) angiography was performed at defined stages during the procedure to exclude coronary damage. Once the corkscrew had been inserted to the correct depth, the corkscrew delivery system was detached from the corkscrew and the system was retrieved. The stent delivery system was introduced over a wire attached distally to the corkscrew. The stent was locked to the corkscrew using the stent delivery system and confirmed engagement of the locking mechanism. The whole system was then tensioned to reshape the TV, promoting annular cinching, and to increase leaflet coaptation under echo guidance. Finally, the stent was deployed in the IVC to maintain the tension applied.
HYBRID APPROACH (chronic group=14 animals)
The hybrid approach was used in chronic animal models to permit evaluation of the safety of the implant and to assess tissue healing and endothelialisation by histological analysis up to 90 days after implantation.
A 10 Fr sheath was placed in the left femoral vein to permit ICE. Through right thoracotomy access, cardiopulmonary bypass with bicaval venous cannulation was established. On the beating heart, the right atrium was opened. The steerable transcatheter device was then introduced through a 24 Fr femoral sheath into the right femoral vein and delivered to the tricuspid annulus. The corkscrew was implanted under direct vision, to confirm the correct implantation. After the closure of the atriotomy and weaning from the cardiopulmonary bypass, the procedure was completed as in the percutaneous approach previously described.
POSTOPERATIVE CARE AND FOLLOW-UP PERIOD
Seventeen animals were sacrificed at the end of the implantation procedure (acute group), while 14 animals were kept alive and followed up for a period up to 90 days post index procedure (chronic group). Each animal was continuously observed in an isolation area during the immediate postoperative period until the animal was breathing comfortably and demonstrating stable vital signs, as determined by the study investigators. Analgesic and antibiotic therapy was administered at the end of the procedure and continued for 10 days. Anticoagulant treatment consisted of Calciparine 16,000 UI/1.6 ml from the day of the procedure until the fourth day after the procedure. Low-dosage aspirin 200 mg/die was administered from the fifth day until the 15th day after the procedure. Upon completion of the follow-up of the chronic study group, the animals were euthanised under general anaesthesia. Post-explant specimens were collected and treated for histological examination.
ECHOCARDIOGRAPHIC AND FLUOROSCOPIC EVALUATIONS
In order to evaluate the ability of the TriCinch system to remodel the TV, the animals were evaluated by ultrasound echocardiography at baseline and post device implantation in the acute study group. The following measurements were assessed: septolateral (SL) diameter in long-axis view, degree of TR, trans-tricuspid peak velocity and leaflet coaptation length (CL). The position of the TriCinch system and the non-displacement of the study device components were assessed by fluoroscopic evaluation. The dimensions of the IVC were evaluated prior to the TriCinch implantation, after the procedure, and at the end of the follow-up period, by IVC angiography, performed by injecting contrast media inside the vessel at the stent implantation level.
POST-MORTEM MACROSCOPIC EVALUATION AND HISTOLOGY
Macroscopic examination was performed on the formalin-fixed specimens. The tricuspid annulus, the valve leaflets and the IVC were examined, and the parameters such as mobility, thrombosis, integration, haemorrhage, stent deployment and macroscopic lesions were evaluated. On each specimen, one X-ray picture was first taken in order to locate the test article in the tissues accurately. The hard part (containing the implant) of the specimen was embedded in resin, whereas the tricuspid annulus, the valve leaflets and portion of the IVC of the specimen were prepared for paraffin embedding. Paraffin sections were stained with Safranin Haematoxylin Eosin (SHE), Masson’s Trichrome (MT) and von Kossa staining, whereas the resin sections were treated by the modified polychromatic Paragon staining method. Qualitative and semi-quantitative histology analysis was performed in the 14 animals of the chronic group.
Statistical analysis
Statistical analysis was conducted using SPSS software, Version 22.0 (IBM Corp., Armonk, NY, USA). Continuous variables are presented as mean±SD and categorical variables are expressed as percentages. Univariable comparisons were performed with a Student’s unpaired t-test for continuous normally distributed data, tested by the Shapiro-Wilk normality test. The Mann-Whitney rank-sum test was used for comparisons of non-parametric continuous data. All p-values <0.05 were considered significant.
Results
FEASIBILITY
The TriCinch device was successfully implanted in all animals (n=31) with both the fully percutaneous and the hybrid approach. In all animals, echocardiographic imaging was sufficient for guiding the procedure to the target tricuspid annulus position. Device implantation time in the fully percutaneous approach (time from insertion of the corkscrew delivery system to the final deployment of the stent in the IVC) was 43.5±20.5 min (range 20-81 min). No damage to the right coronary artery was observed at angiography in any implantation (Figure 3). No animal required post-implant inotropic support and, in all the implanted animals, fluoroscopy showed the integrity of the device at the end of the procedure.
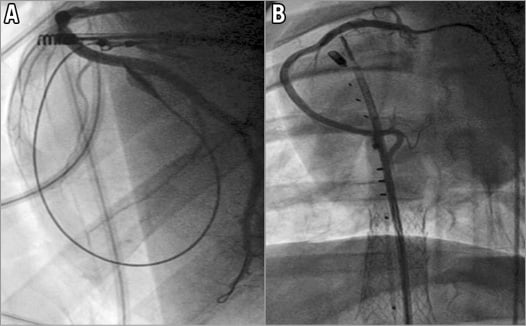
Figure 3. Examples of right coronary artery angiography. A) Post corkscrew implantation. B) Post procedure.
Post-mortem macroscopic evaluation of the explanted heart showed that the intended target annular zone was reached in all the animals treated with the fully percutaneous approach.
EFFICACY OF THE DEVICE (ACUTE GROUP)
The efficacy of the implant to promote TV remodelling was evaluated in the 17 animals of the acute study group (Figure 4, Figure 5).
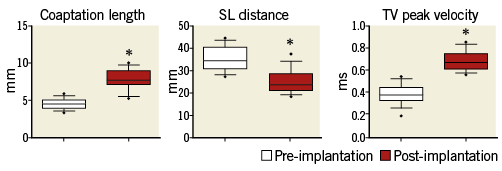
Figure 4. Box-plot presentation of relevant tricuspid valve parameters measured by echocardiography before and after the reduction of the annular size by the device (n=17 animals of the acute group). Each box represents 10-90 qq data with “•” being the outliers. Statistically significant (p<0.001) results were marked with an asterisk (“*”).
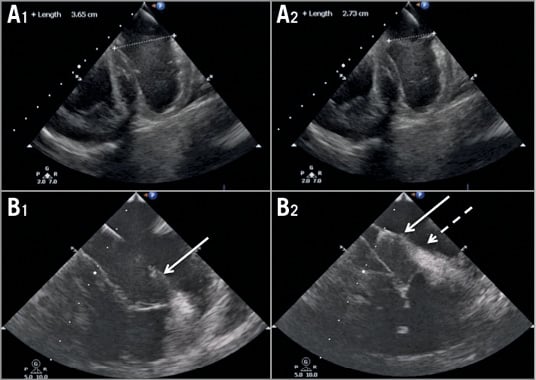
Figure 5. Echocardiography demonstrating the position of the implanted system and its corresponding effect on septolateral distance prior to (A1-B1) and after (A2-B2) application of tension.
ANNULAR REMODELLING AND LEAFLET COAPTATION
As a result of TriCinch implantation, the SL dimension decreased from 35.2±5 mm to 24.8±5 mm (p=0.001). Leaflet coaptation significantly improved: CL increased from 4.5±0.7 mm to 7.78±1 mm (p=0.001).
DOPPLER EVALUATION OF TV
As a result of annular remodelling, mean trans-tricuspid peak velocity increased from 0.38±0.1 to 0.68±0.1 (p=0.001). Due to the absence of TR at baseline (healthy animals), TR severity reduction could not be evaluated in this model.
CHRONIC SAFETY AND MORPHOLOGIC EVALUATION (CHRONIC GROUP)
The chronic safety of the implant was evaluated in the 14 animals of the chronic study group. All 14 animals survived the chronic follow-up time of 90 days. No major adverse events were observed during follow-up. Fluoroscopic evaluation confirmed the non-displacement and the correct positioning of the device in all the chronic cases. The distance between the screw and the stent remained stable at follow-up in all the animals.
MACROSCOPIC EVALUATION
At gross anatomy examination of the TriCinch device, healing of the corkscrew was sufficient, with tissue in-growth and fibrous covering of the device. The corkscrew showed good tissue integration, tight fixation and correct site of placement, with a correct and firm insertion within the tissue, in the anterior portion of the tricuspid annulus (Figure 6). In addition, gross examination did not show migration of the anchor or of the stent or damage to them or to the tissue as a result of contraction. The stent was found to be fully expanded and tapering to the IVC wall in all the specimens analysed. No stent dislocation was observed.
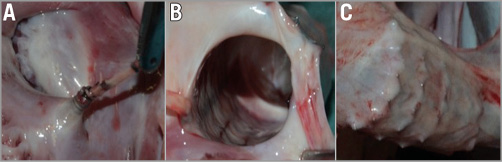
Figure 6. Macroscopic evaluation. The macroscopical analysis showed the integrity of the test system with fibrotic encapsulation of the corkscrew (A) and the stent (B) with preserved integrity of the vena cava (C).
The tension over the Dacron band appeared preserved, with the locking mechanism showing signs of tissue integration, consistent with the follow-up evaluation.
No damage was caused to the native tricuspid annulus, tricuspid leaflets, the IVC or to adjacent structures, including the atrial and ventricular wall and right coronary artery. Gross anatomy findings of animals implanted with the TriCinch system revealed that all the components of the implant were well healed and covered by fibrous tissue. Biocompatibility of the device was appropriate as it did not cause any thrombus formation, toxicity or hyperplasia/hypertrophy.
HISTOLOGICAL ASSESSMENT
The macroscopic analysis showed the corkscrew firmly attached to the TV annular tissue. The Dacron band and stent did not exhibit abnormalities except some signs of stent protrusion. No thrombus deposit was noted in any of the specimens. The histopathological analysis confirmed a slight to moderate tissue integration of the corkscrew and stent components with no undesirable local inflammatory reaction. The Dacron band was encapsulated without signs of undesirable inflammatory reaction. Histological analysis of the surrounding tissue did not show significant change at the level of the myocardial tissue, tricuspid annulus, IVC or valve leaflets analysed for any of the specimens (Figure 7).
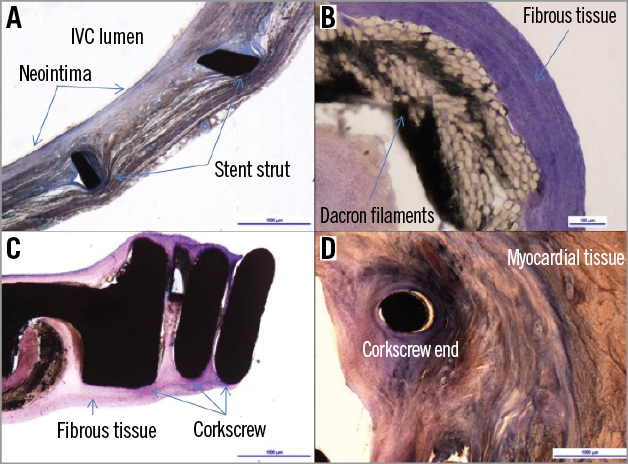
Figure 7. Histology: representative images of histological preparations. A) The stent was integrated into the surrounding tissue of the inferior vena cava, with a minor to moderate macrophagic and lymphocytic reaction around the struts. A slight neointimal growth was noted. B) The Dacron band was mostly covered by a fibrous tissue showing a thin interfacial macrophagic and giant cells layer. C) Luminal portion of the corkscrew enclosed by fibrotic tissue. D) A minor macrophagic and lymphocytic reaction was observed around the fixation screw that was inserted into the APC tissue. Bordering myocardial tissue did not show signs of pathological changes.
Discussion
Significant FTR occurring late after left-heart surgery is observed in up to 40% of patients and is associated with reduced survival, decreased exercise tolerance, poor quality of life and increased hospitalisation for heart failure3-5,14. However, only a minority of these patients undergo TV surgery, as reoperation for recurrent TR carries an in-hospital mortality of up to 37%6-8. This results in a large number of untreated patients with TR. In recent years TR has become an emerging health problem and consequently interest has been growing in the pathophysiology and treatment of FTR. Therefore, percutaneous procedures may be an attractive alternative to surgery for patients deemed to be high-risk surgical candidates. Different new devices and approaches are currently under preclinical development, but so far clinical data are still inconsistent for native TV15,16.
This study demonstrates the safety and feasibility of the percutaneous implantation of a sutureless device designed for TV remodelling under beating heart conditions in a preclinical large animal model.
Annular dilatation and tricuspid leaflet tethering with lack of coaptation are the most important pathophysiologic determinants concurring in FTR17. Therefore, the current surgical treatment of FTR consists of resizing and remodelling the tricuspid annulus by either ring or suture annuloplasty. In case of excessive leaflet tethering, which is a marker of an advanced phase of the disease, some authors suggest a more aggressive valve remodelling addressed towards correcting valve tethering and increasing leaflet coaptation18,19.
The basic principle of the transcatheter repair technique here reported is to remodel the tricuspid annulus by reducing the septolateral distance using the mechanism of annular cinching, in a manner which permits the most mobile anterior leaflet to coapt to the opposite leaflet, and counteracting annular dilatation. The efficacy of tricuspid valve remodelling by annular cinching has been reported in the surgical setting by Hetzer et al who described the so-called “double orifice valve technique” to correct TV incompetence in case of advanced leaflet tethering and annular dilatation19.
The concept of using a corkscrew anchor is commonly applied for pacemaker and defibrillator lead fixation20,21. Recently, the feasibility of the implantation of a fully percutaneous placement of a sutureless direct annuloplasty band in the mitral position by means of anchor fixation elements has been reported22. The corkscrew is designed to allow a safe and precise attachment to the tissue of the tricuspid annulus and to ensure retrievability and repositionability.
Significantly, our study results elucidate the favourable safety profile of the TriCinch device: in the chronic study group no death or adverse events were observed during the entire follow-up period. Implant of the corkscrew under direct vision allowed a standardised implant in all the animals of the chronic group, in order to avoid any bias due to different angle and depth of tissue penetration, which could have affected the tissue-device interaction. Post-explant gross examination and histological analysis showed that the components were well integrated and no migration of the device was observed. Examination of tricuspid leaflets from all implanted animals in this study was unremarkable. Similarly, no device-related injuries were observed in the surrounding structures, including the IVC, from any animal in the study, and no right coronary artery injury was observed in the study.
Regarding efficacy, this study was conducted in animals with normal TV and no or minimal TR; therefore, we were not able to document TR severity reduction. However, the data of the present study showed that TV remodelling is achievable with TriCinch implantation. The device was able to promote annular dimension reduction and an increase in leaflet coaptation length. Significant transvalvular velocity was observed in all animals following treatment, demonstrating significant valve remodelling. These observations are very promising when considering the potential clinical use of this device. In particular, surgical experience suggests that obtaining a good surface of leaflet coaptation is particularly important to ensure efficacy and durability of the repair23. Intracardiac echocardiography integrated with fluoroscopic imaging was sufficient to guide the procedure in all cases; however, live 3D echocardiography (which is not possible in the animal model) should improve imaging guidance in the human experience.
Although the procedure has been successful in the animal model, several challenges have to be overcome. It should be recalled that this study has been conducted in healthy animal models and that further studies are needed to test the device in a diseased animal model to confirm the safety and efficacy of the procedure in the pathological anatomy. In particular, the angulation of the annulus in relation to the IVC requires careful consideration in the presence of severe right atrial enlargement. Another important issue which should be considered before clinical application is that in chronic TR the IVC is often severely dilated; therefore, precise anatomical evaluations have to be carried out to choose the correct stent size, preventing eventual IVC injuries.
Limitations
The major limitation of the study is that the device has been tested in a healthy animal model, with normal tricuspid valve function. Another limitation is that the distance between the anchor and the stent may potentially be affected by deep breathing. This issue has not been quantified in this animal model, which has been developed mainly to assess the feasibility and the safety of the procedure.
Conclusions
Percutaneous beating-heart remodelling of the tricuspid annulus with the TriCinch device is feasible and safe in acute and chronic healthy animal models. Future studies are needed to test the device in a diseased animal model to confirm safety and efficacy in a pathologic setting. The presented percutaneous approach may, in the future, be an alternative to open surgical procedures in high-risk patients. Moreover, it could be used concomitantly with transcatheter mitral valve repair procedures to treat associated FTR through a fully percutaneous approach.
| Impact on daily practice Transcatheter treatment of tricuspid valve disease represents the next frontier of structural heart interventions. This preclinical study shows for the first time the feasibility and the safety of a new percutaneous annular remodelling system to treat functional tricuspid regurgitation in a large animal model. The results of this preclinical study were instrumental in allowing the move towards clinical practice with a first-in-man trial. |
Conflict of interest statement
A. Latib, P. Denti, K. Lynn, A. Addis and A. Guidotti declare a financial relationship with 4Tech Cardio. H. Vanermen and F. Maisano are co-founders of 4Tech Cardio. The other authors have no conflicts of interest to declare.

