
Abstract
Aims: The aim of the study was to report preclinical safety and feasibility of a new transcatheter direct mitral annuloplasty intervention in an acute animal model.
Methods and results: Twenty healthy pigs underwent Cardioband (Valtech Cardio, Or Yehuda, Israel) transcatheter implantation under intracardiac echocardiographic and fluoroscopic guidance. Through a neo inferior vena cava approach, transseptal access was arranged. The device was delivered into the left atrium using a multi-steerable catheter and fixed to the mitral annulus with multiple helix anchors. Following device cinching, reduction of annular size was evaluated. In all animals the device could be successfully implanted and displayed 100% function, with the average procedure duration and fluoroscopy times being 78±23 minutes and 27±9 minutes, respectively. In total, 246 anchors (average 12.3 per device) were delivered and optimal anchor placement was achieved in 95.1%, while inadequate anchor position (4%) and injury of the coronary sinus or atrium (0.8%) occurred in the rest. Following maximal cinching, diastolic transmitral flow velocity and coaptation lengths were markedly increased (p<0.001), whereas septolateral and intercommissural distances were significantly decreased (p<0.001), when compared to pre-contraction baseline, demonstrating efficient annular reduction by the device.
Conclusions: Transcatheter direct annuloplasty with a surgical-like adjustable device is feasible in the porcine animal model. The humanised porcine model has been instrumental in demonstrating feasibility and in establishing the procedural steps.
Introduction
Transcatheter mitral valve interventions are a valuable alternative to surgery for high-risk and inoperable patients with mitral regurgitation (MR)1,2. Currently, MitraClip® (Abbott Vascular, Santa Rosa, CA, USA) therapy is the most commonly performed transcatheter mitral repair procedure1. Although good clinical outcomes have been associated with MitraClip therapy, complete MR abolition is obtainable only in a minority of patients. The absence of concomitant annuloplasty has been advocated as a potential explanation3.
Annuloplasty is a standard surgical procedure performed either as a stand-alone therapy (mostly in case of functional MR), or as an adjunct to leaflet repair in patients with degenerative MR4. Surgical mitral annuloplasty has been associated with improved early outcomes and longer repair durability in patients with degenerative MR5. Lack of annuloplasty is an independent factor for recurrent MR, particularly in patients with more advanced mitral valve disease and annular dilatation6. A reliable transcatheter annuloplasty could provide a desirable adjunct to percutaneous leaflet repair. Furthermore, transcatheter annuloplasty could also be used as a stand-alone procedure in selected patients with functional mitral regurgitation. Herein we report the preclinical acute outcomes with a transcatheter direct annuloplasty device, the Cardioband system (Valtech Cardio, Or Yehuda, Israel), designed to implant a “surgical-like” annuloplasty ring with a transseptal approach on the beating heart under fluoroscopic and echocardiographic guidance7.
Methods
THE ANIMAL MODEL
The present series is a subgroup of animals from a larger series previously reported8. The peculiarity of this subgroup is that all the animals underwent complete transcatheter implant of the Cardioband system with subsequent full cinching of the device after the implantation. In particular, the study population is composed of 20 consecutive healthy white female adult pigs (104±9 kg). All the procedures were performed under intracardiac echocardiography (ICE) and fluoroscopic guidance, in two institutions equipped with hybrid operation rooms. The animal model followed standardised protocols in both institutions.
The animal studies were conducted in compliance with Italian Ministry of Health national law (116/92), European Union guideline (86/609/EEC) and Swiss Federal animal protection law and ordinance (licence ZH 138/2010).
After loss of postural reflexes following premedication with ketamine (20 mg/kg), azaperone (1.5 mg/kg) and atropine (0.75 mg), the anaesthesia was deepened by a bolus injection of propofol (1-2 mg/kg BW) and the animals were intubated. Anaesthesia was then maintained with 2-3% isofluorane and propofol (2-5 mg/kg/hr). Amiodarone (2-3 mg/kg bolus IV) was administered to stabilise the heart rhythm. Pain management included fentanyl constant rate infusion (0.02 mg/kg/hr) for the duration of the procedure.
Under general anaesthesia, vital parameters and ECG monitoring were continuously observed and recorded. Six Fr and 10 Fr sheaths were placed in the femoral artery and vein, respectively, for blood pressure monitoring, post-procedural coronary angiogram and intracardiac echocardiography.
In order to mimic a human implantation position in this porcine model, a neo inferior vena cava was surgically implanted, as previously described8. Briefly, after additional local anaesthesia (intercostal block), a right thoracotomy was performed and the pericardial sac was incised, exposing the right and left atrium and the inferior vena cava. A 10 mm Dacron tube graft was anastomosed to the right atrium. The graft was oriented with a 45° angle to the midline of the animal, to reproduce a “human-like” angulation. A large bore sheath with a haemostatic valve was then fixed at the opposite end of the tube graft to serve as an access point for the further interventional steps. Transseptal access was gained through the neo inferior vena cava approach.
For the introduction of the 9 Fr ICE catheter (ViewFlex™ Xtra; St. Jude Medical, St. Paul, MN, USA), a 10 Fr introducer sheath was positioned in the left ventricle through the apex through a surgical subxyphoid incision. The apical probe was used to achieve long-axis images of the mitral valve. A second ICE catheter was introduced from the left femoral vein and positioned in the right atrium to guide the transseptal puncture and to provide short-axis views of the annulus. Heparin was administered at 250 IU/kg and repeated if the activated clotting time was shorter than 300 s until the completion of the experiment.
At the end of the procedure, after a left coronary angiogram had been performed to rule out coronary damage, the animals were euthanised by overdose of Na-pentobarbital and the hearts were explanted for subsequent analysis.
THE CARDIOBAND SYSTEM
The Cardioband is a variable length tubular Dacron band delivered over the posterior circumference of the annulus and implanted with multiple helical stainless steel anchors (Figure 1).
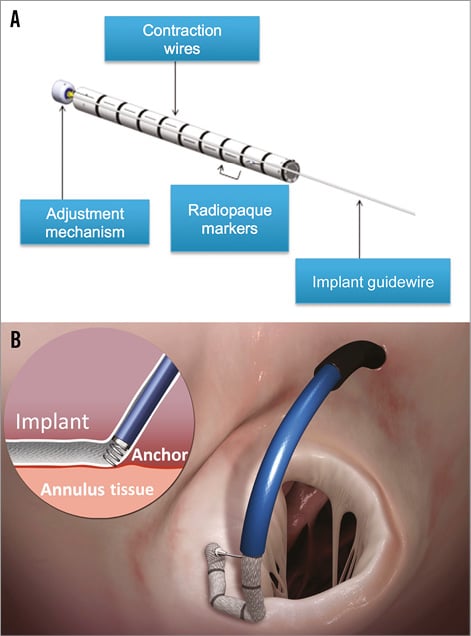
Figure 1. The Cardioband device. A) The Cardioband implant, which is a variable length tubular Dacron band. Several radiopaque markers (one every 8 mm) allow its visualisation under fluoroscopy. The implant contraction wire inside the Dacron band allows the cinching. B) The implant is delivered over the posterior circumference of the mitral annulus, on the atrial side, and fixed with multiple helical anchors.
The Cardioband delivery system (CDS) consists of the implant delivery system (IDS) and the 24 Fr transseptal steerable sheath (TSS). The IDS comprises a steerable guide catheter (GC) and an implant catheter (IC) with the Cardioband implant mounted on its distal portion. A set of helicoidal anchors is used to fix the implant to the native annulus (one anchor every 8 mm of mitral annular circumference). Each anchor is repositionable and retrievable until release from the torque transmission cable.
The anchors are delivered from inside the Cardioband to eliminate the risk of anchor embolisation and to simplify the delivery. After the placement of all of the anchors, the IDS is removed and cinching is performed by using a size adjustment tool (SAT), which is inserted and engaged to the adjustment mechanism of the implant. The spool is rotated to reduce the Cardioband length. Once the required implant size is reached, the SAT is disengaged, the tension on the contraction wires is locked and the implant guidewire is removed.
The implant technique
DEVICE IMPLANT
Using the neo inferior vena cava graft as access, a standard transseptal system with a Brockenbrough® curved needle (Medtronic, Minneapolis, MN, USA) was used to cross the interatrial septum under intracardiac echocardiography (ICE) guidance. Following heparin administration, a 0.035” Amplatz Super Stiff™ wire (Boston Scientific, Marlborough, MA, USA) was advanced into the left atrium. The TSS was then advanced over the wire until it reached the middle of the left atrium. Then it was deflected to approximately 90° and turned towards the mitral valve. The IDS was then advanced until the tip of the delivery system was at the level of the anterolateral commissural area, on the atrial side. An 0.0018” wire emerging 4 mm from the side of the implant was used to guide the device towards the anterolateral commissure. Echocardiography was used as follows: short-axis projections were used to identify the delivery system location around the annulus, while long-axis projections were used to visualise the relative position of the device to the annulus, to follow the anchors’ deployment and to prevent damage to adjacent structures (e.g., leaflets, circumflex artery, atrial wall, coronary sinus). The first anchor was then implanted in correspondence to the anterolateral commissural annulus by gentle rotation of the knob in the delivery system under echocardiographic and fluoroscopic guidance (Figure 2A). Before anchor release, a pull test was done to ensure anchor fixation. If correctly implanted, then the anchor would be released. The tip of the implant was then positioned posterior and medial to the first anchor, along the mitral annulus and a second anchor was implanted (Figure 2B). These manoeuvres were performed repeatedly until the posteromedial commissure was reached and the last anchor delivered (Figure 2C). After implanting all the anchors, the implant was released from the IDS and the catheter removed, while keeping the TSS in place. Only a wire remained connected to the implant-cinching mechanism. Over this wire, the cinching tool (SAT) was advanced and connected to enable the post-implant cinching.
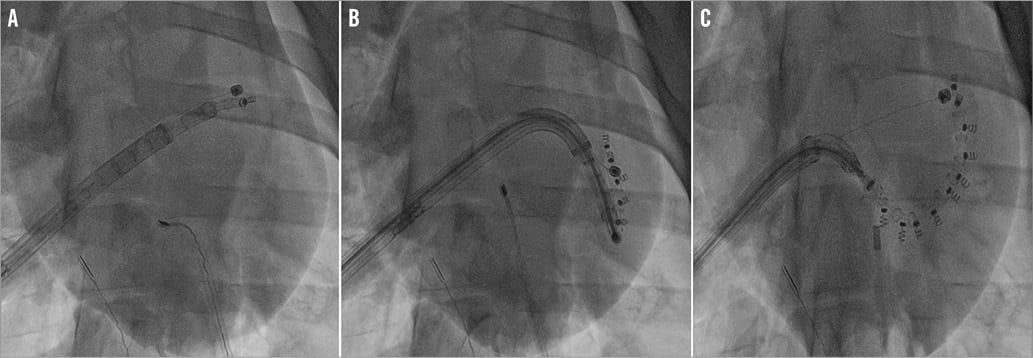
Figure 2. Fluoroscopic procedural steps of the Cardioband implantation. After crossing the interatrial septum, the TSS was advanced to the middle of the left atrium and steered towards the mitral valve. The IDS was then progressed until the tip of the delivery system was at the level of the anterolateral commissural area. A wire emerging 4 mm from the side of the implant served to guide the device towards the anterolateral commissure and to provide stability for the first anchor implantation (A). By gentle manoeuvring of the TSS and the IDS under fluoroscopic and echocardiography guidance, the implant delivery system was moved along the mitral annulus and subsequent anchors were placed (B) at 8 mm intervals in order to attach the annuloplasty implant to the tissue, until the posteromedial commissure was reached (C).
ANNULAR CINCHING
Cinching was performed in all animals under ICE guidance. Since the animals had normal mitral valve function, the efficacy of cinching was tested by determining the achievable amount of annular reduction and the creation of a diastolic gradient. For this reason, the Cardioband was cinched until maximal contraction was achieved. Echocardiographic analysis of mitral valve parameters such as diastolic septolateral and intercommissural distance, leaflet coaptation length, annulus surface area as well as maximal pressure gradient and blood velocity over the valve were performed at this point. Using the SAT, the Cardioband was returned to baseline dimensions to allow post-mortem examination of the location of the anchors. The SAT was then disconnected and retrieved.
FLUOROSCOPIC IMAGING
Due to the different orientation of the heart relative to the chest cavity in the animal model, a slightly caudal anteroposterior projection is equivalent to the left anterior oblique projection in humans. Lateral projections in the animal are equivalent to right anterior oblique projections in humans.
DATA COLLECTION AND ANALYSIS
Data on procedural time, anchor implantation time, total fluoroscopy time and implantation success were collected. Procedural time was determined as time elapsed from transseptal puncture to the deployment of the last anchor. Anchor implantation time was defined as the duration between implantation of the first and the last anchor. Total fluoroscopy time was defined as the time (minutes) under effective fluoroscopy for each implant. Successful implantation was defined as a full deployment (from commissure to commissure) of the Cardioband device in the posterior annulus achieved, and a commissure-device gap (distance between the commissure and the first anchor) of less than 1 cm.
Procedural adverse events were recorded during the implantation. Upon explant, gross pathological examination was performed and relevant observations were documented, including distance of implant from annulus and lesions of surrounding structures.
Results
Twenty healthy female adult pigs were successfully implanted with the Cardioband system (Table 1).
The transseptal access was achieved, and the delivery system was advanced into the left atrium in all animals without complications. The device was successfully implanted in all animals, with a mean procedure time of 78±23 minutes and a mean total fluoroscopy time of 27±9 minutes. A total of 246 anchors were implanted, with a successful anchor placement rate of 95.1%. Implantation time from first to last anchor was 52±19 minutes, on average 6±2 minutes per anchor.
Complete pre- and post-cinching echo quantitative assessment was available for 12 animals. Following Cardioband cinching, a marked (p<0.001) decrease in septolateral (–8.2±6.4 mm) and intercommissural distance (–9.6±3.5 mm) was observed. Moreover, a significant (p<0.001) increase in coaptation length (3.8±1.6 mm) and pressure gradient (2.4±1.9 mmHg) over the mitral valve was recorded (Figure 3).
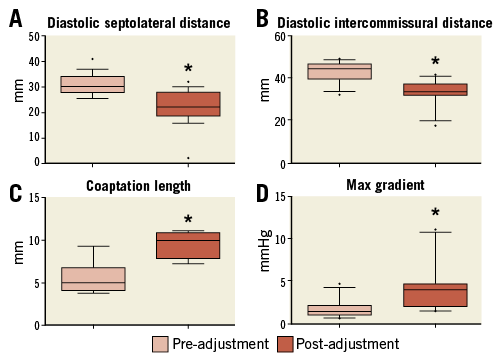
Figure 3. Box-plot presentation of relevant mitral valve parameters measured by intracardiac echocardiography (ICE) after the implantation of the Cardioband as well as after reduction of the annular size by device contraction (cinching) (data available for 12/20 animals). In particular, panels A and B show the reduction of septolateral and intercommissural distance of the mitral annulus, respectively. Panel C shows the variation in coaptation length and panel D the increase of peak transmitral gradient. Each box represents 10-90 percentile range with “•” being the outliers. Statistically significant (p<0.001) results were marked with an asterisk (*).
Procedural adverse events and observations at post-mortem examination are listed in Table 2.
Discussion
By using multimodality imaging guidance in an animal model which was specifically designed to test “human grade” devices with an endovascular approach, percutaneous transcatheter mitral annuloplasty with the Cardioband system was successfully completed in all animals. Echocardiographic quantification showed that device cinching significantly reduced mitral annulus diameter and increased coaptation length and maximal pressure gradient over the mitral valve in the 12 animals assessed.
The MitraClip device in particular has been successfully used to reduce MR in diverse pathology settings9. Although early outcomes are promising, lack of annuloplasty could explain some early failures and durability issues10.
Different annuloplasty ring devices are commonly used in surgical practice (complete or incomplete, rigid, semi-rigid or flexible), and there is no consensus regarding the best technique for surgical annuloplasty: surgeon preference and experience are the main determinants in the choice of the device. The Cardioband system is the first device developed to closely reproduce surgical mitral valve annuloplasty by fixing a flexible incomplete Dacron band to the annulus, using multiple helix anchors, on a beating heart. As the anchors, like surgical sutures, are placed 8 mm apart, the total number of anchors, and procedural duration for that matter, are strongly dependent on the size of the annulus.
The animal model chosen to prove the Cardioband concept was the healthy adult swine. Since annuloplasty is an established technique for mitral repair, the animal model was mainly designed to demonstrate feasibility of a transcatheter, transseptal implant of a surgical-like annuloplasty device in the mitral annulus, under echocardiographic and fluoroscopic guidance (Figure 4). Of note, the Cardioband size can be decreased and increased as long as the SAT is connected.
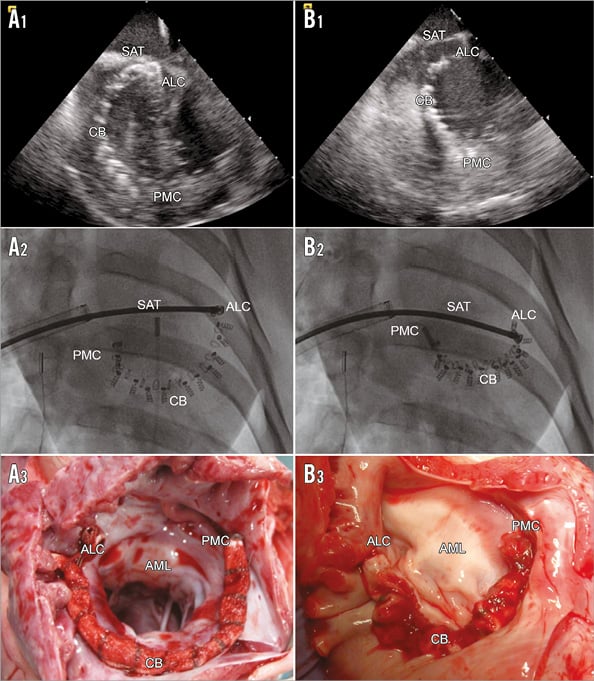
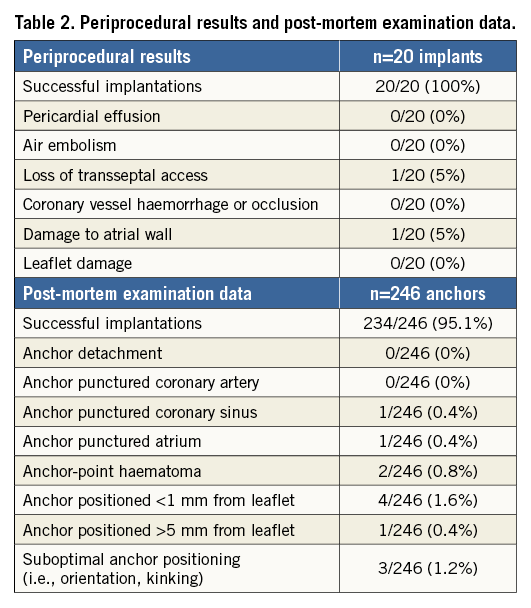
Figure 4. ICE short-axis, fluoroscopic and native post-mortem, representative images of the fully implanted uncinched (A1 to A3) and cinched (B1 to B3) Cardioband system. ALC: anterolateral commissure; AML: anterior mitral leaflet; CB: Cardioband; PMC: posteromedial commissure; SAT: size adjustment tool
Transcatheter mitral valve therapies require precise imaging for guidance. While fluoroscopy remains mandatory to monitor device manipulation, echocardiography is necessary to identify the left heart structures. Currently, live 3D echocardiography is the gold standard imaging modality for mitral interventions, including MitraClip therapy11,12. This remains a challenge in the large porcine model, as TEE imaging of the mitral valve could be obscured by an interposed lung lobe.
In contrast to previously described difficulties13, transfemoral and transapical ICE for long- and short-axis views of the mitral valve were successfully used to guide the procedure in this study (Figure 5). The device and all relevant cardiac structures were clearly visible in both fluoroscopic and ICE imaging, resulting in high marks in visibility (Figure 2).
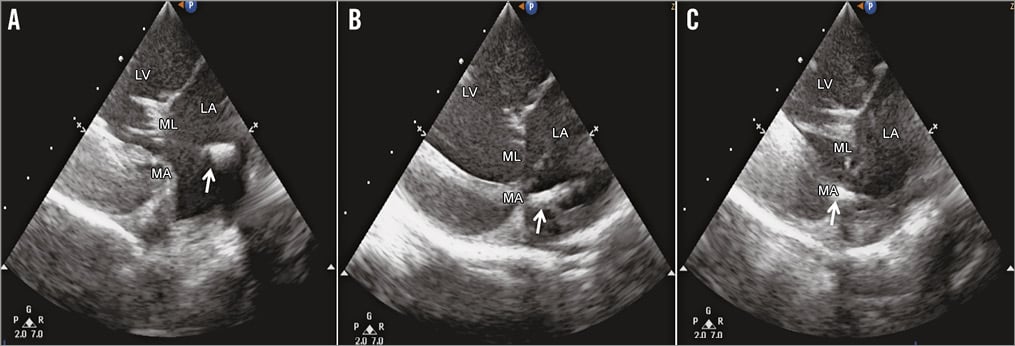
Figure 5. Intracardiac echoradiography of the Cardioband implantation procedure. Intracardiac echoradiography guidance of the Cardioband implantation procedure in a large animal model is essential in order to achieve optimal positioning. The delivery system was first oriented towards the leaflets (A) and, after correction, properly positioned on the mitral annulus (B), where the anchor was deployed in the tissue (C). White arrow indicates the tip of the delivery system (A & B) and the anchor implanted in the MA in C. LA: left atrium; LV: left ventricle; MA: mitral annulus; ML: mitral leaflet
Limitations
Due to the limitations of the healthy animal model, the therapeutic efficacy of the Cardioband could not be demonstrated in this study. Although complete annuloplasty is considered the gold standard for stand-alone annuloplasty in functional MR, a posterior band configuration for the device was preferred for safety reasons, since the risk of aortic valve injury was felt to be a potential issue when designing the transcatheter approach. Moreover, the main determinant for repair durability after annuloplasty in functional MR is the achievement of good leaflet coaptation rather than the type of ring used14,15. Reduction of SL and IC length, as well as increase in maximal pressure gradient and leaflet coaptation length after cinching of the annuloplasty band, suggest that the Cardioband induces changes in annular geometry which are similar to a surgical implant. Another limitation is that complete pre- and post-echocardiographic quantification was available for only 12 animals.
Conclusions
In conclusion, the Cardioband system is the first transcatheter device developed to closely reproduce surgical annuloplasty. This study demonstrated that transseptal beating heart implantation of an adjustable annuloplasty device is safe and feasible in the animal model. This animal model has been instrumental in developing the procedural steps and in preparing the implanting physicians for the first-in-man trial.
| Impact on daily practice The Cardioband system is the first transcatheter device developed to closely reproduce surgical annuloplasty. In particular, the humanised porcine model has been fundamental to set the procedural steps before the first-in-man trial. |
Conflict of interest statement
F. Maisano, A. Guidotti and P. Denti are consultants for Valtech Cardio. The other authors have no conflicts of interest to declare.

