
Abstract
Aims: The aim of this study was to test the feasibility of transcatheter mitral valve implantation of the NaviGate device in acute and chronic preclinical models.
Methods and results: We evaluated NaviGate valved stent implantation in the mitral position in an acute swine model (n=24, ≤5 days) through three different approaches - transatrial, transapical, and transseptal - and in a chronic swine model (n=12, >10 days) through a transatrial approach. The NaviGate implantation procedures were successful in 83% of the acute model studies (n=20) and 83% of the chronic model studies (n=10). Echocardiographic assessment showed low gradient across the valved stent (mean gradient <3 mmHg) and the left ventricular outflow tract (mean gradient <6 mmHg). Post implantation, there was no mitral regurgitation (MR) in 75% (n=15) of the acute studies and mild MR in 25% (n=5). In the chronic model, there was no MR in 60% (n=6) and mild MR in 40% (n=4). The implantation procedure was aborted in four acute studies due to inferior vena cava injury and in two chronic studies due to prosthesis-annulus mismatch.
Conclusions: In preparation for clinical application, transcatheter mitral implantation of the NaviGate valved stent was proved feasible in acute and chronic preclinical models. The three featured delivery approaches are of particular value for high-risk patients with functional MR and challenging vascular access.
Abbreviations
LA: left atrium
LVOT: left ventricle outflow tract
MA: mitral annulus
MR: mitral regurgitation
MV: mitral valve
PVL: paravalvular leakage
TAVR: transcatheter aortic valve replacement
TMVR: transcatheter mitral valve replacement
VD: valve dislodgement
Introduction
Mitral regurgitation (MR) is the most common valvular heart disease in the United States of America with a 1.7% prevalence of a moderate to severe degree in the general population1. Although there has been an increased MR prevalence in the advanced age population with comorbidities or impaired left ventricular (LV) function, surgical referral has been more frequently denied, as surgery is considered high risk2,3.
The successful implantation of a valved stent in the aortic position using a catheter-guided approach without assistance of cardiopulmonary bypass introduced new alternatives for high-risk patients4-6. Transcatheter mitral implantation has recently been a new focus of experimental research. Several devices and delivery systems of transcatheter mitral valve replacement (TMVR) are in development; however, this approach is still in its infancy7-10. Certain technical aspects render TMVR clinical application challenging, including the anatomical site, disease pathology, and complexity of the mitral valve apparatus, as well as vascular access and navigation.
The NaviGate™ valved stent (NaviGate Cardiac Structures, Inc., Lake Forest, CA, USA) was developed to address these technical aspects with a unique delivery feature that allows transcatheter implantation via three different approaches. We demonstrated the feasibility of the new valved stent in a preclinical model ahead of the intended first-in-man TMVR clinical trial.
Materials and methods
NAVIGATE VALVED STENT
The NaviGate device is a trileaflet valve fabricated from equine pericardium and mounted on a self-expanding tapered nitinol stent (Figure 1). Equine pericardium has the advantages of decreased thickness, superior flexibility, and increased tensile strength in comparison with other available materials such as bovine pericardium, and has been fixed with low concentrations of buffered glutaraldehyde. The NaviGate valved stent has three unique features: the cone configuration with a low height profile of 21 mm, which produces a diffuser effect to minimise transvalvular gradients and the possibility of LV outflow tract (LVOT) obstruction; the anchoring mechanisms of the stent to the mitral annulus (MA); availability in different sizes (36 mm, 40 mm, 44 mm, 48 mm, and 52 mm) to fit in different mitral annulus sizes. The anchoring mechanism involves 12 radially arranged winglets on the atrial component of the stent that engage the MA with a support of woven microfibre polyester fabric lining. Additionally, 12 radially arranged graspers on the ventricular component of the valved stent engage the mitral leaflets and the subvalvular apparatus within the left ventricle (Figure 2). This design secures the stent to the MA, prevents dislodgement to the left atrium during systole, minimises paravalvular leakage, and avoids coronary vessels obstruction. Compression of the circumflex coronary artery is a concern with excessive oversizing of the valved stent to the MA size. Our preference is to oversize the device by only 5-10% to the annulus size to achieve better sealing.
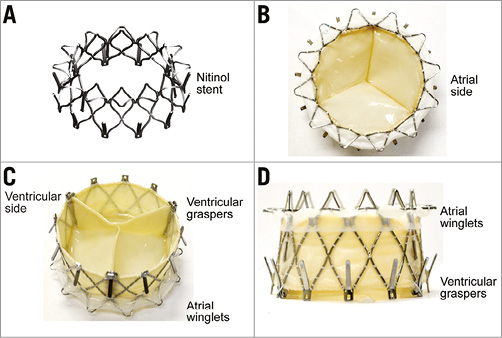
Figure 1. The NaviGate valved stent. A) Nitinol stent frame. B) Inflow (LA) view. C) Outflow (LV) view. D) Side view.
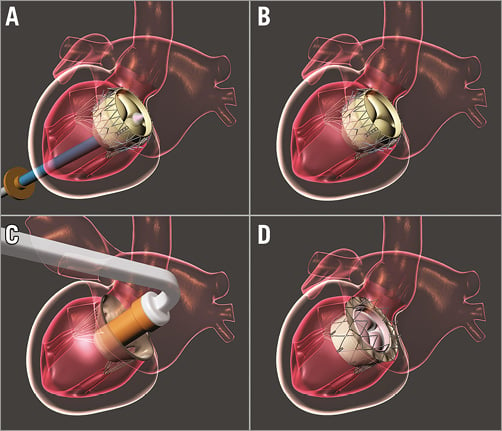
Figure 2. Mechanism of fixation: the atrial winglets engage the mitral annulus and the ventricular graspers engage the mitral leaflets and the subvalvular apparatus. A) & B) Transapical implantation. C) & D) Transatrial implantation.
The NaviGate delivery system for the acute studies consists of a catheter with a central conduit accommodating a 0.038” guidewire and has an 18 Fr shaft reinforced with interior metallic coiling. The distal section is flexible and includes the 30 Fr stent-enclosing capsule and a mechanism that allows steering in four directions and depth control by forward or reverse motion of the distal capsule when in the immediate vicinity of the MA plane (Figure 3). The system for chronic studies evokes the configuration of a mitral commissurotomy device. The curved shape and angulation of the shaft leads the distal end into a central alignment position of the MA. The size of the distal capsule is identical to the acute delivery system (Figure 4). Retraction of the capsule causes exposure of the distal aspect of the valved stent deploying the ventricular graspers that capture the leaflets above the mitral chordae tendineae.
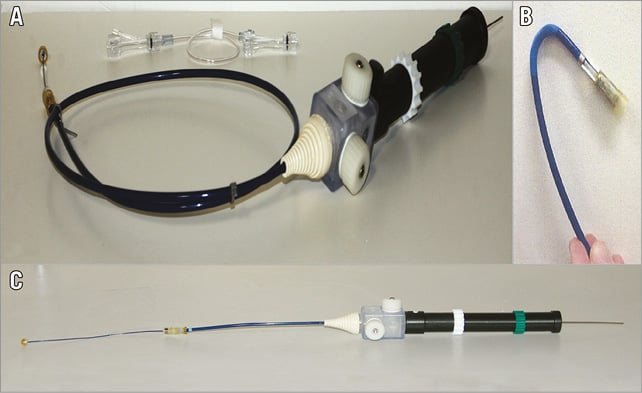
Figure 3. NaviGate valved-stent implantation delivery system. A) & B) Acute study transseptal delivery system. C) Acute study transapical and transatrial delivery system.
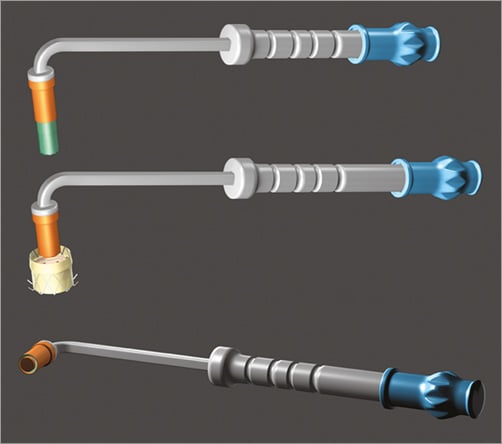
Figure 4. Chronic study transatrial delivery system.
EXPERIMENTAL DESIGN
ACUTE STUDY
The acute study duration ranged between one and five days per protocol. The study ended with fluoroscopic imaging of the valved stent position and echocardiographic assessment of valve function. The animal was then euthanised and the valved stent examined in situ for any damage. NaviGate valved stent implantation was planned in 24 swine for acute studies, using transatrial (n=7), transapical (n=9), and transseptal (n=8) approaches.
CHRONIC STUDY
The chronic study was designed to assess the durability of the valved stent after implantation in the mitral position. The initial experiments have shown that the transatrial pathway is the simplest and safest implantation approach and thus it was chosen for carrying out this study. The NaviGate valved stent implantation was planned in 12 swine that were followed to assess midterm haemodynamic function, durability, and pathological changes of the valved stent leaflets such as pannus formation, calcification, and/or thrombosis.
IMPLANTATION PROCEDURE
All animals received humane care in compliance with the Animal Welfare Act & Public Health Services protocols. The study protocol was approved by the Institutional Animal Care & Use Committee at the Gateway Innovation Laboratory, Shanghai, China. The size of the valved stent used in each experiment was determined by preoperative computed tomography scans as well as intracardiac echocardiograms. No rapid pacing is required for implantation of the NaviGate valved stent as the device functions during a slow controlled release when it reaches 60% of its opening diameter. During implantation, the valved stent is released from one end (atrial or ventricular) to the opposite end. For transatrial and transseptal implantation, the valved stent is released from the ventricular end to the atrial end to allow the graspers to engage the mitral leaflets and the subvalvular apparatus within the LV. For transapical implantation, it is released from the atrial end to the ventricular end to allow the winglets to engage the MA. Intracardiac and/or transoesophageal echocardiography were used for baseline evaluation before, during, and after the surgical procedures. Echocardiographic, haemodynamic, and valve function assessments were repeated after 20 minutes of haemodynamic stability. The current valved stent is no longer recapturable after the ventricular winglets come off the delivery system. A new delivery system is under development with the feature of recapturing the device after delivery.
TRANSATRIAL APPROACH
The surgical procedure involves the use of left thoracotomy through the fourth intercostal space. In the acute study, a 32 Fr NaviGate introducer sheath (12 cm) was placed in the LA using the Seldinger technique (Figure 3C). In chronic studies, the LA wall was opened and the new NaviGate delivery system (Figure 4) was placed, de-aired, and flushed. Using echocardiographic and fluoroscopic guidance, the valved stent-mounted delivery system was centred over the mitral valve orifice and positioned with equal valve height above and below the MA. The valved stent was deployed and anchored to the MA. Next, the delivery system was safely withdrawn, the LA puncture access site was closed using a 4-0 Prolene purse-string suture, and then the left thoracotomy was also closed in the usual fashion.
TRANSAPICAL APPROACH
The surgical procedure involves the use of the lower sternotomy or left thoracotomy through the fifth intercostal space. Using the Seldinger technique, a 32 Fr NaviGate introducer sheath (12 cm) was placed for retrograde access to the MA (Figure 3C). The valved stent was navigated and deployed under echocardiographic and fluoroscopic guidance. Next, the delivery system was safely withdrawn, the LV puncture access site was closed using a purse-string suture, and the incision closed in the usual fashion.
TRANSSEPTAL APPROACH
After sequential dilation of the interatrial septum access site, a 32 Fr NaviGate introducer sheath (18 cm) was placed for antegrade access to the MA. Then the delivery system with the NaviGate device (Figure 3A, Figure 3B) was introduced into the LA through a transseptal puncture created in the interatrial septal wall. The valved stent was navigated and deployed under echocardiographic and fluoroscopic guidance.
DATA ANALYSIS
Haemodynamic measurements after NaviGate stented valve implantation were summarised using standard descriptive statistics and are reported as mean±standard deviation.
Results
ACUTE STUDY
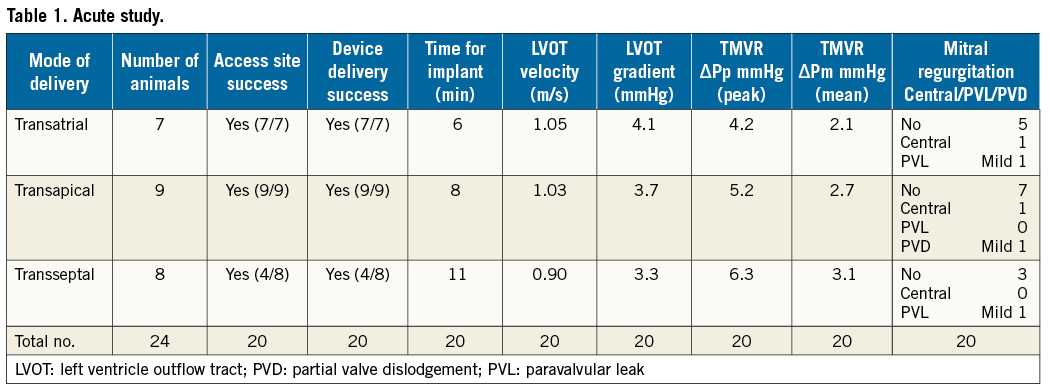
Using the three delivery modes (transseptal, transatrial, and transapical), the NaviGate valved stent was successfully implanted in 20 of the 24 animals (83% of the acute study). Four transseptal studies were aborted due to inferior vena cava injury. The follow-up varied from one to five days per protocol. Fluoroscopic and echocardiographic assessment confirmed good function of the new mitral valve with accurate alignment and secure anchoring of the valved stent onto the MA (Figure 5, Figure 6). In all cases, there was no LVOT obstruction, pericardial effusion, or encroachment on coronary arteries, along with preservation of the subvalvular apparatus. The entire procedure time in all three approaches of the acute studies ranged from 20 to 45 min, and the device deployment time ranged from 1 to 3 min.
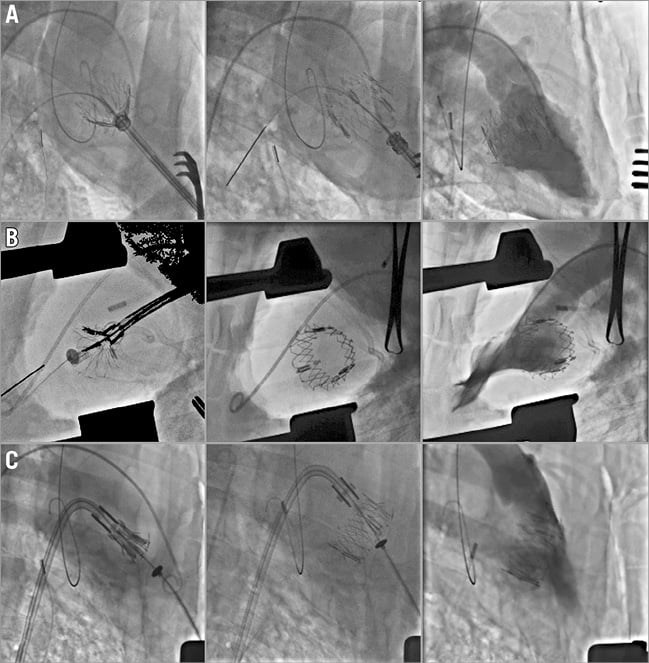
Figure 5. Acute study LV angiogram. A) Transapical. B) Transatrial. C) Transseptal.
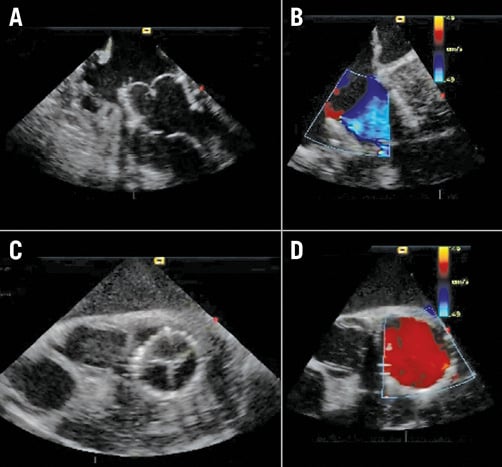
Figure 6. Acute study echocardiography. A) Long-axis view. B) Colour Doppler in long axis view. C) Short axis view. D) Colour Doppler in short-axis view.
TRANSATRIAL DELIVERY (N=7)
There was successful implantation in all animals. Time for the device delivery at the MA after the surgical access to the left atrium was six minutes. There was no MR in five animals; one animal had central MR and one animal had mild paravalvular leak (PVL). The valve function evidenced normal haemodynamic performance and normal pressure gradient across the implanted valve (mean 2.1 mmHg). Mean gradient across the LVOT was 4.1 mmHg.
TRANSAPICAL DELIVERY (N=9)
There was successful implantation in all animals. Time for the device delivery at the MA after the surgical access to the left atrium was eight minutes. There was no MR in seven animals; one animal had central MR, and one animal had mild partial valve dislodgement due to prosthesis-annulus mismatch. The dislodgement can be attributed to the undersized valved stent (40 mm) that was deployed in a larger mitral annular diameter (≥45 mm). The valve function evidenced normal haemodynamic performance and normal pressure gradient across the implanted valve (mean 2.7 mmHg). Mean gradient across the LVOT was 3.7 mmHg.
TRANSSEPTAL DELIVERY (N=8)
There was successful implantation in four animals while the procedure was aborted in four animals for inferior vena cava injury. Time for the device delivery at the MA after the surgical access to the left atrium was 11 minutes. There was no MR in three animals; one animal had mild PVL. Valve function evidenced normal haemodynamic performance and normal pressure gradient across the implanted valve (mean 3.1 mmHg). Mean gradient across the LVOT was 3.3 mmHg (Table 1).
CHRONIC STUDY (≥10 POSTOPERATIVE DAYS)
The follow-up varied from 10 to 50 days per protocol. Ten of 12 animals survived the intervention; two died on the table, one from severe MR and the other one from total valve dislodgement (VD), both due to prosthesis-annulus mismatch. Of the remaining 10 animals, one animal died at 24 days due to cardiac arrhythmias, and one animal died at 54 days due to pneumonia. The other eight animals were euthanised per designated protocol - two animals after 10 days, two animals after 24 days, two animals after 30 days, and two animals after 43 days. Fluoroscopic and echocardiographic assessment confirmed good function of the new valved stent with accurate alignment and secure anchoring of the valved stent into the MA (Figure 7A-Figure 7C).
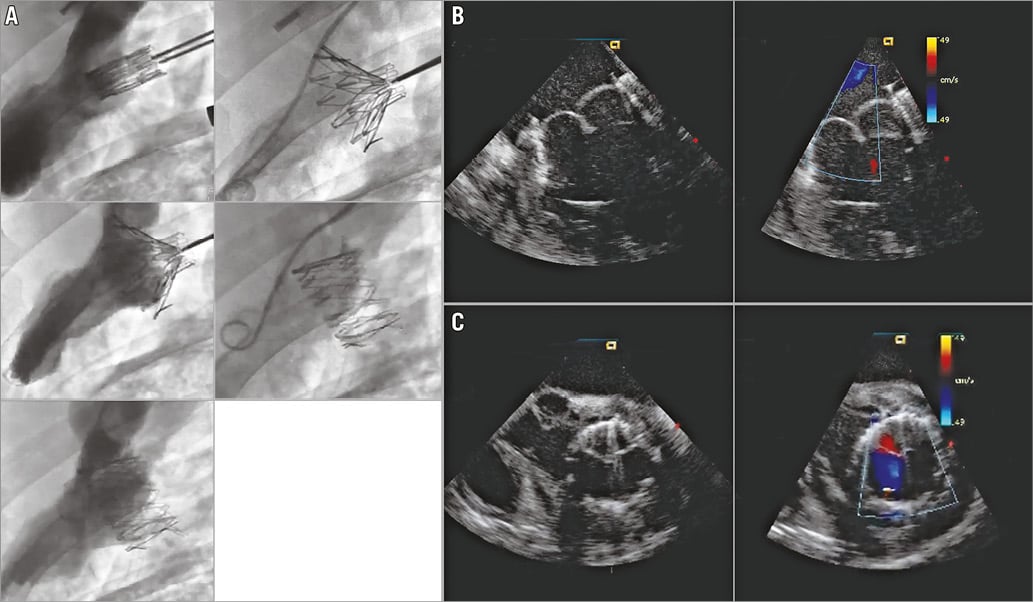
Figure 7. Transatrial NaviGate valved-stent implantation in the mitral position. A) Chronic study LV angiogram. B) Chronic study echocardiography, long-axis view. C) Chronic study echocardiography, short-axis view.
Conformity to the MA with no PVL or central MR was observed in six valves, while one mild PVL and three mild central MR were observed. In all cases, there was no LVOT obstruction, pericardial effusion, or encroachment on the coronary arteries, along with preservation of the subvalvular apparatus. Valve function evidenced normal haemodynamic performance and normal pressure gradient across the implanted valve, as well as LVOT gradients (Table 2).
The mean cardiac output was 3.3±1.6 L/min. There was no significant difference between the mean left atrial pressure and the LV end-diastolic pressure (13.5±3.5 vs. 11.2±2.6 mmHg), or across the LVOT (LV pressure 76.7±11.5 vs. ascending aorta pressure 73±10.6 mmHg). The entire procedure time was in the same range as the acute-term study.
POST-MORTEM EXAMINATION
After euthanasia, the heart was excised and weighed. A gross examination showed a proper anchoring and alignment of the valve in all the animals. There were no signs of wear or damage to the anchoring winglets. Of particular interest, the chronic tissue response to the valved stent showed normal response and evidence of tissue ingrowth without thrombus formation. There was a complete circumferential seal in both sides of the valved stent without evidence of device-related trauma (Figure 8A-Figure 8C). An X-ray cell irradiator (Faxitron, Tucson, AZ, USA) was used to visualise the position of the valved stent within the MA in the resected heart which showed no distortion or fracture of any of the segments of stent in the acute or chronic study.
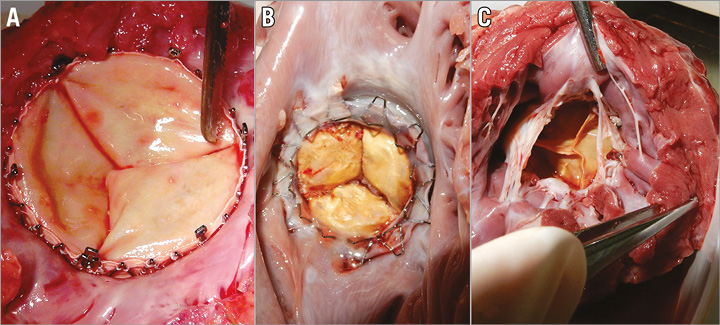
Figure 8. Post-mortem examination. A) Acute study inflow view. B) Chronic study inflow view. C) Chronic study outflow view.
Discussion
The development of a clinically applicable percutaneous mitral device is more challenging compared to transcatheter aortic valve replacement (TAVR). Several technical aspects are particular to the mitral valve (MV) as the most complex heart valve and, consequently, certain considerations are required for successful TMVR11-14. The TAVR device is deployed with a sutureless implantation technique into a well-defined circular and calcified aortic valve that helps the new valved stent firmly anchor in a proper and stable position, and only needs to oppose the diastolic arterial pressures during valve closure15. The MV is a complex apparatus in which the new prosthetic valve has to anchor in a very loose and non-circular saddle-shaped annulus. In addition, it has to interact with the moving parts of the MV during the cardiac cycle, such as mitral leaflets, chordae, papillary muscles, and the LV posterior wall that induces sphincter contraction reducing the MA. All of these create a very unstable and technically challenging environment for secure valve fixation16,17.
The NaviGate valved stent was designed with particular attention to overcoming the technical challenges associated with TMVR. The feasibility of the NaviGate valved stent was tested in a preclinical model with overall satisfactory results. Echocardiography showed no significant post-implantation MR or high transvalvular gradients. There was no obstruction of the LVOT, coronary arteries, or subvalvular apparatus. Moreover, implantation of the NaviGate valved stent was successful through the three described approaches– transapical, transfemoral, and transatrial. We believe that the mitral valved stent should be larger than the native MA (oversizing may be inevitable) to achieve good anchoring of the valve within the MA. Rather than fight the MA geometry, the mitral valved stent should dictate a new MA dimension. The combination of a low profile and truncated cone self-expanding stent configuration also provides very beneficial haemodynamic parameters, low intrusion into the left atrial space, reducing (if any) PVL, and is better resistant to high dislodgement forces such as the LV dP/dt. These are the features that differentiate the NaviGate from the existing valved stents in the field. The equine pericardium is superior to other available materials such as bovine pericardium in terms of decreased thickness, superior flexibility, and increased tensile strength. Another advantage is availability in terms of quantity and cost. Durability is a concern of equine pericardium as with any other bioprosthesis; however, it was not suggested in the in vitro testing or clinical follow-up of the Medtronic 3f Enable® aortic valve (Medtronic, Minneapolis, MN, USA)18,19.
An important limitation of this study is the use of healthy animal models where the MA is not enlarged with a very low atrial roof that precludes substantial extension of the valved stent into the LA. The healthy pig differs widely from the patient presenting with functional MR. The pig has a hypertrophied LV and small-chambered heart which renders navigation into the MV apparatus not a simple task. The animal ventricle pumps strongly generating a dP/dt that possibly exceeds those of the normal human (~2,000 mmHg/sec at rest) and definitely far exceeds the dP/dt of the patient presenting with functional mitral regurgitation (FMR) (~700 mmHg), so configuration of stent design is critical to absorb these forces without stent damage. The need to achieve a good measurement of the MA as well as good visualisation during deployment is critical to avoid mismatch of the valved stent to the native MA and valve migrations over time.
At this time, the NaviGate valved stent has been successfully implanted in five patients, two in the mitral position and three in the tricuspid position. Several devices have been developed for transcatheter mitral implantation and tested in animal models, and clinically13,14,20. Despite substantial technical progress in the development of valved stent designs, most of the emerging transcatheter mitral devices remain in their early development stages21-23. However, they will ultimately need to be proven safe and effective when compared to the surgical gold standard. While technically and surgically demanding, this technology may become an important alternative in a selected group of patients with high operative risk and a low probability of long-term successful MV repair24.
TMVR-associated early mortality could be attributed to application in high-risk patients with multiple comorbidities, or further impairing LV function by perforating a diseased myocardium through the transapical approach25. The next frontier for valve surgery is the development of a transcatheter-delivered mitral valved stent feasible for clinical application that requires catheter delivery into the LA via a transseptal approach or minimally invasive surgical access to the LA.
Limitations
An important limitation is the use of healthy animal models with normal cardiac anatomy and physiology. Another limitation is the difficulty of applying advanced imaging technology in an animal laboratory.
Conclusions
Mitral regurgitation has a high prevalence in older patients. For those with significant comorbidities and associated high surgical risk, transcatheter mitral replacement is emerging as an alternative option to treat MR. This preclinical evaluation in acute and chronic preclinical models has shown that transcatheter implantation of the self-expanding NaviGate valved stent is feasible and safe, and results in a secure and stable engagement of the MA, with a well-functioning valve. The three delivery options suggest potential ease of implantation for high-risk patients, particularly for those with challenging vascular access.
| Impact on daily practice Transcatheter valve therapy has been progressively evolving to provide alternative options for high-risk surgical patients. In this work, we have tested the feasibility of the NaviGate valved stent in an animal model in preparation for clinical application. Three delivery approaches are of particular value for high-risk patients with functional MR and challenging vascular access anatomy. |
Funding
The study was funded by NaviGate Cardiac Structures, Inc.
Conflict of interest statement
J. Navia is the inventor on patents related to this device, and is a consultant to NaviGate Cardiac Structures, Inc. (NCSI). R. Quijano is an inventor on patents related to this device, and holds stock in NCSI. K. Thyagarajan, and R. Bertwell work at NCSI. The Cleveland Clinic has an ownership position on patents licensed to and holds stock in NCSI. The other authors have no conflicts of interest to declare.

