
Abstract
Aims: The aim of this study was to evaluate the impact of two different transcatheter mitral valve replacement (TMVR) fixation strategies on the neo left ventricular outflow tract (neo-LVOT) and aorto-mitral angulation (AMA) after TMVR.
Methods and results: Two different self-expanding nitinol valved stents were developed for transapical TMVR. In one group, the stents were fixed with an annular fixation system (ANN group, n=6). These prototypes were compared with an apical tether fixation TMVR system (AP group, n=11) in another group. Echocardiographic evaluation of the AMA and the neo-LVOT was conducted before and one hour after implantation. Maximal and minimal AMA (AMAmax and AMAmin) during the cardiac cycle of the AP group were significantly narrower than those of the ANN group (AMAmax: 39±8° vs 67±15°, p<0.001, AMAmin: 33±10° vs 56±22°, p=0.009). More severe reduction of the neo-LVOT diameter was observed in the ANN group (60±11% vs 26±14%, p<0.001). The ANN group had a higher peak velocity through the neo-LVOT post implantation (200±52 cm/s vs 108±15 cm/s, p<0.001).
Conclusions: The apical fixation system maintains a smaller and more stable aorto-mitral angulation and a larger neo-LVOT, thereby reducing the risk of postoperative neo-LVOT obstruction in this experimental setting.
Introduction
Transcatheter mitral valve replacement (TMVR) has been developing at an unprecedented rate since the world’s first-in-man on-pump TMVR in June 2012 in Copenhagen, Denmark1. One year later, the first in-man off-pump transapical TMVR was performed by our working group2. In recent years, to investigate the feasibility of the TMVR procedure in high-risk patients with severe symptomatic mitral regurgitation, clinical feasibility trials of different types of TMVR prosthesis have been carried out3. Meanwhile, a considerable number of cases using transcatheter aortic valve replacement (TAVR) prostheses in the mitral position within surgical rings (valve-in-ring), failed mitral bioprosthetic valves (valve-in-valve) or in cases of severe mitral annular calcification (valve-in-MAC) have been reported4,5,6,7,8,9,10.
However, new challenges still arise, especially for cases with a native mitral valve. The adjacent relationship between the mitral valve complex and the left ventricular outflow tract (LVOT) may be of particular importance. It has been revealed with cardiac computed tomography (CT) that the device itself and the native anterior mitral leaflet (AML) may cause LVOT obstruction (LVOTO). An increasing number of LVOTO cases have been reported and LVOTO after TMVR has gained more recognition in the literature11,12.
The concept of the neo-LVOT has emerged during recent years and was considered a predictive factor for LVOTO after TMVR12,13. The neo-LVOT is created by the prosthesis, the AML, and the interventricular septum. Theoretically, in addition to prosthesis-related factors, aorto-mitral angulation (AMA), the thickness of the basal septum and the left ventricular size are the main factors which influence the neo-LVOT dimensions.
In the present study, we tested two mitral valved stent prototypes for the TMVR procedure. In one group, the valved stents were fixed in the mitral annulus by an apical tether fixation system (AP group) which has been previously presented by our group14,15,16. In the other group, the valved stents were anchored by an annular fixation system (ANN group). The aim of this study was to evaluate and compare the effects of the two fixation systems on the AMA and the neo-LVOT in an in vivo porcine model.
Methods and material
MITRAL VALVED STENTS
In the ANN group, the mitral valved stents consisted of a modified D-shaped atrial cuff and a D-shaped annular stent body which was made with an additional 20 annular lateral struts to achieve secure systolic annular fixation. This was previously tested in vitro by our working group, using an in vitro force-measurement system17. The height of the ventricular element was 13.6±3.6 mm and the short axis was 28.7±1.6 mm (26-30 mm) in width. To reduce the risk of any paravalvular leakage (PVL), the stent was covered with a polytetrafluoroethylene membrane (Figure 1). A commercially available trileaflet bioprosthetic valve or a native bileaflet valve (produced by D. Simionescu, University of Clemson) was sewn into the ventricular stent body.
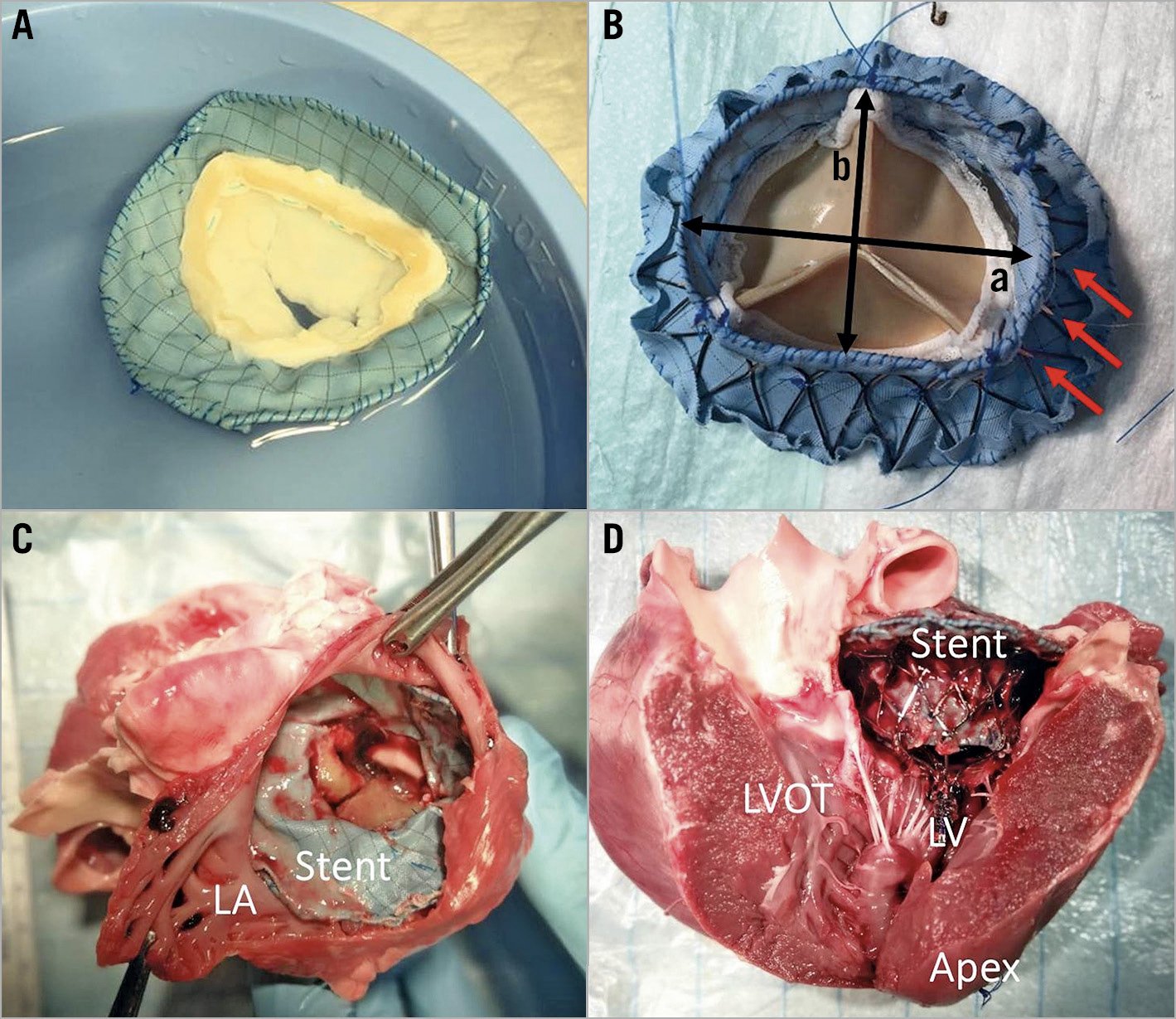
Figure 1. The D-shaped mitral valved stent (in vitro and after implantation in vivo). A) An atrial view of the bileaflet mitral valved stent of the ANN group. B) Ventricular view of a trileaflet valved stent of the ANN group. a) Inter-commissural length of ventricular part of the mitral valved stent of the ANN group was 32-36 mm. b) Short-axis length of ventricular stent body of the ANN group was 28-30 mm (red arrows: the annular fixation struts on the outside of ANN valved stent). C) Top view of implanted mitral valved stent of the AP group. D) Lateral view of the implanted mitral valved stent within the native mitral annulus of the AP group. The ventricular part of the mitral valved stent faces towards the apical fixation system on the apex. LA: left atrium; LV: left ventricle
In the AP group, the valved stents also included an atrial cuff and a ventricular body. The mitral valved stent consisted of a double-frame ventricular element. The outer frame of the ventricular element was designed to match the D shape of the mitral orifice. The D-shaped outer frame gradually bent downwards into a circle until connecting to the bottom of the inner frame. The diameter of the circular inner frame together with the bottom outer ring of the valved stent was on average 28 mm. It was designed to support the trileaflet bioprosthetic valve. The sizes of the mitral valved stents ranged from 26 to 30 mm and were selected according to the native mitral orifice for the stent fitting the mitral annulus. The average height of the stents of the AP group was 15.9±4.8 mm and the diameter of the ventricular body ring was 28.6±0.8 mm. The stent was also covered with a polytetrafluoroethylene membrane. A single apical tether connected to an apical epicardial fixation pad which was attached to the bottom of the stent for anchorage of the overall mitral valved stent (Figure 1).
For the two study groups, two self-expanding nitinol mitral stents were produced by RTM Inc., Medical Group, Graben-Neudorf, Germany.
PORCINE IN VIVO MODEL AND MITRAL VALVED STENT IMPLANTATION
Twenty female pigs of the German Landrace and Edelschwein breeds or their cross-breeds underwent transapical off-pump mitral valved stent implantation (ANN group: n=10, average body weight: 48±2 kg, AP group: n=12, average body weight: 47±3 kg, p=n.s.). All animals received humane care in compliance with the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources, revised in 2011. The experimental transapical TMVR procedure via a lower ministernotomy has already been described in previous studies of our working group14,15,16,17,18.
MEASUREMENTS
Echocardiographic evaluation of the aorto-mitral angle (AMA), the LVOT diameter (LVOTd) and Doppler-derived peak flow velocity through the LVOT (Vmax) and mitral valve inflow velocity were recorded and analysed before and one hour after implantation using 2D and 3D transoesophageal echocardiography (TEE). The presence of PVL was also evaluated and recorded after implantation.
The AMA and the LVOTd were measured at two points in time of the cardiac cycle. First was at the end of isovolumic systole or the beginning of isobaric systole, which is the beginning of ventricular ejection. The maximal LVOTd (LVOTdmax) and the minimal AMA (AMAmin) can be observed during this phase of systole. The second measurement was at the end of isobaric systole, which is the point in time to record the minimal LVOTd (LVOTdmin) and the maximal AMA (AMAmax). In this study, the LVOTd was measured as the shortest distance between the ventricular stent bottom rim and ventricular wall using 2D TEE in the left ventricular long-axis view. The AMA was defined as the angle formed by the centre axis of the aortic annulus and the mitral annulus before implantation. After stent implantation, it was defined as the angle between the centre axis of the aortic annulus and the tube-shaped ventricular element of the stent (Figure 2).
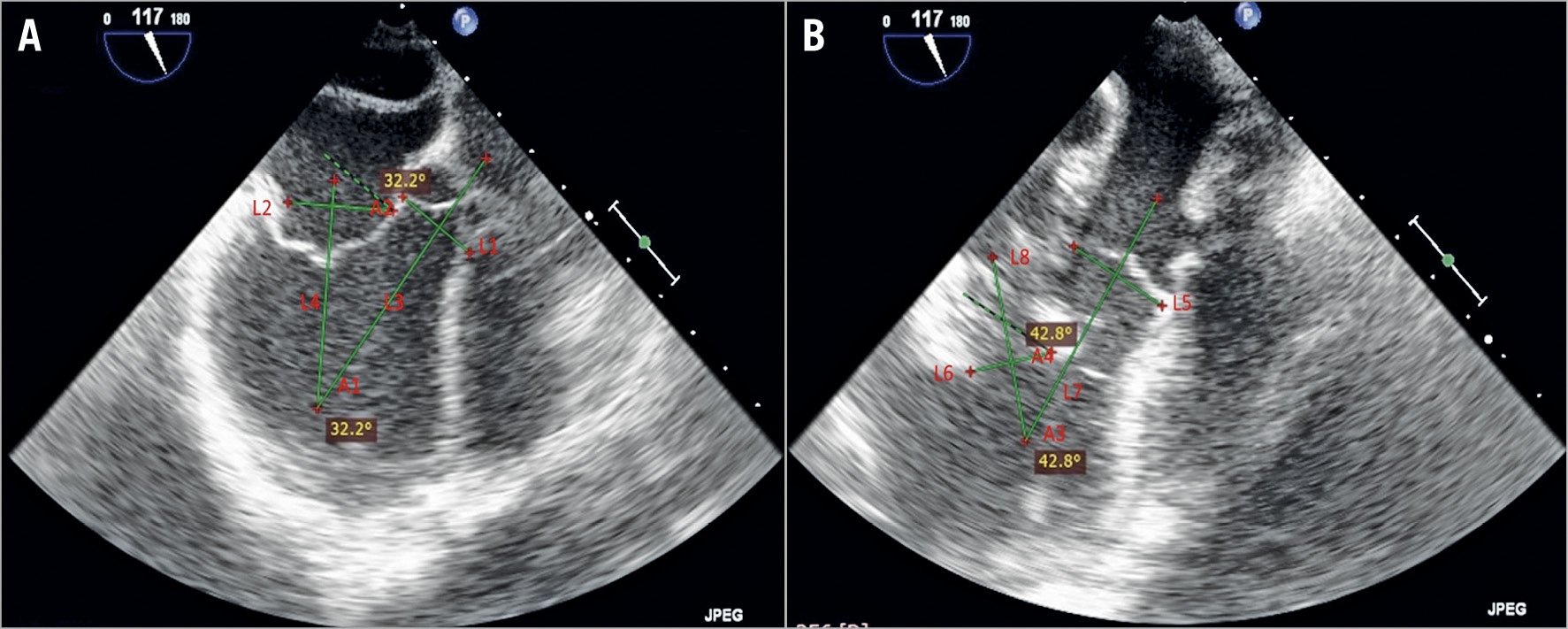
Figure 2. TEE measurement before (A) and after (B) stent implantation. L1 or L5: projection line of the aortic annulus. L3 or L7: central axis of LVOT. L2: projection line of the mitral annulus. L4: central axis of the mitral annulus. L6: projection of the bottom rim of the stent. L8: central axis of the stent. A1: preoperative AMA. A3: postoperative AMA. A2: sharp angle between L1 and L2. A4: sharp angle between L5 and L6. In terms of geometry, A1=A2 and A3=A4. In this study, we measured A2 and A4 to obtain the value of the preoperative and postoperative AMA.
STATISTICAL ANALYSIS
All statistical analyses were performed using the statistical software package SPSS, Version 23 (IBM Corp., Armonk, NY, USA). Continuous variables are expressed as mean and standard deviation and categorical data as count and percentages. Changes in echocardiographic parameters from baseline after TMVR within the two groups were evaluated with the dependent t-test for paired samples. Inter-group comparisons were conducted with the Student’s t-test or Mann-Whitney U test for two independent samples. The probability of a type I error was set to 5% (α=0.05).
Results
The main results are summarised in Table 1 and Table 2.
Seventeen animals successfully underwent the TMVR procedures. In addition, one animal of the AP group and two animals of the ANN group died prior to stent implantation due to ventricular fibrillation. Two stents in the ANN group were malpositioned into the left atrium or into the left ventricle. These two animals were excluded from this study.
All animals had structurally normal hearts with no mitral or aortic valve stenosis or regurgitation. Among the 17 animals which underwent a successful TMVR procedure, peak inflow velocity across the valved stent after implantation was 120±9 cm/s in the ANN group and 108±33 cm/s in the AP group (p=0.42), reflecting a normal mitral valve inflow gradient (Table 2). Mild PVL occurred in 3 animals of the ANN group and 4 animals of the AP group. Additionally, trace PVL was observed in 2 of 11 animals of the AP group. All subsequent animals demonstrated no PVL. Valvular leakages were not observed in the overall cohort, except two trace leakages in each of the study groups.
INTRA-GROUP COMPARISON
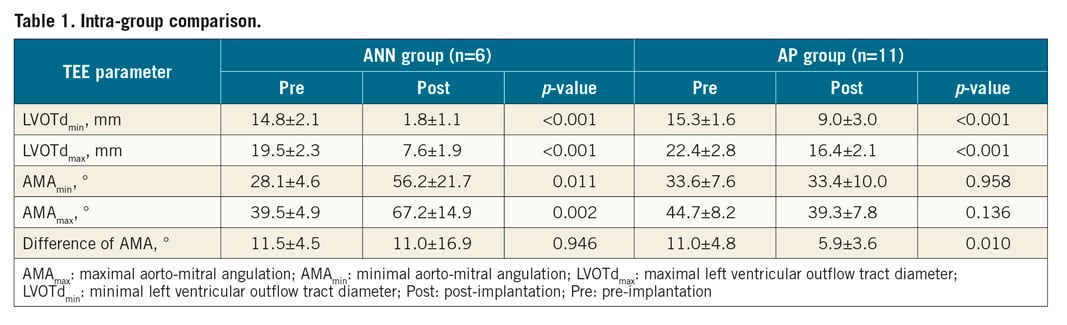
In the ANN group, echocardiographic evaluation revealed an enlarged AMA at both points in time after stent implantation (AMAmax: p=0.002, AMAmin: p=0.011), while no significant differences for the post-implantation AMA data were observed in the AP group compared to the pre-implantation data. Furthermore, in the AP group, the difference between AMAmax and AMAmin decreased slightly after implantation (p=0.01). In both groups, the neo-LVOTd was smaller than the native LVOTd at both measurement points (p<0.001).
In the ANN group, the neo-LVOTd at the end of isovolumic systole was only 39.8±11.5% of the native LVOTd, while in the AP group the neo-LVOTdmax/LVOTdmax was 74.5±13.6%. In contrast to the laminar LVOT flow at baseline, the peak velocity through the LVOT after implantation was slightly elevated in the ANN group at 200±52 cm/s. No relevant flow acceleration in the LVOT after TMVR was noted in the AP group (108±15 cm/s).
INTER-GROUP COMPARISON
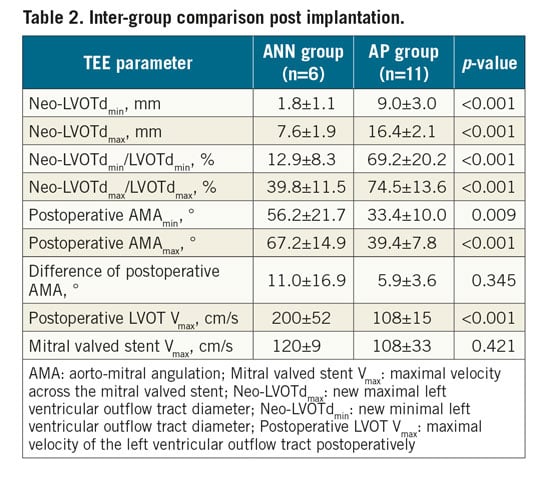
After stent implantation, both the AMAmax and the AMAmin of the AP group were significantly smaller than those of the ANN group (AMAmax: p<0.001, AMAmin: p=0.009). Accordingly, compared to the AP group, more severe reduction of neo-LVOTd was observed in the ANN group, regardless of the absolute value or ratio (p<0.001). The ANN group had a higher peak flow velocity through the neo-LVOT post implantation (p<0.001) (Table 2, Figure 3).
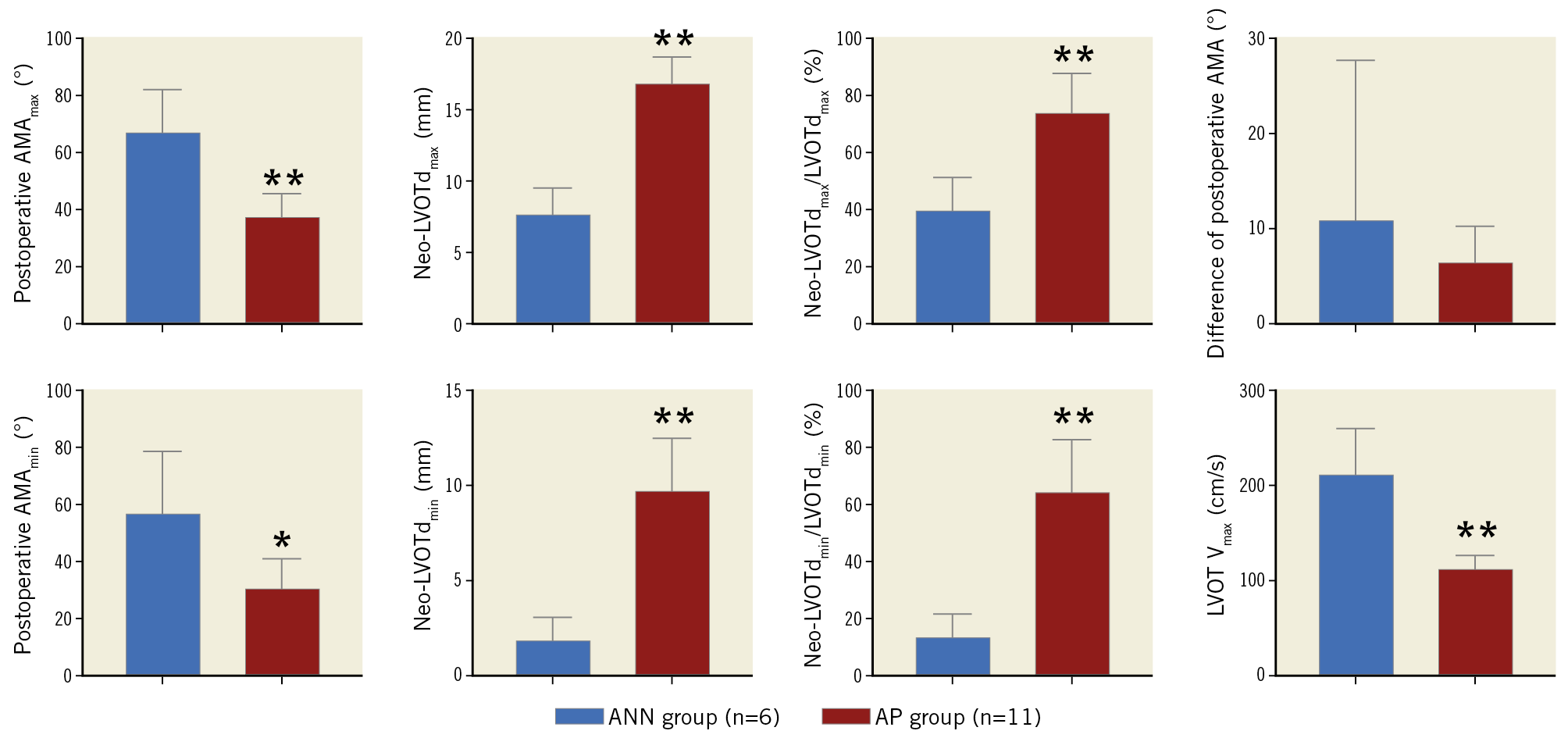
Figure 3. Inter-group comparison of echocardiographic evaluations (ANN group in blue, and AP group in red). * p<0.01; ** p<0.001. All values are mean±SEM.
Discussion
In these porcine experiments we demonstrated for the first time that apical fixation of the mitral valve prosthesis in native mitral valves results in a smaller postoperative aorto-mitral angle (AMA) in comparison to annular fixation. Therefore, apical fixation is more favourable for achieving a larger neo-LVOT and reducing the risk of LVOT stenosis. This might have been due to the perpendicular implantation of the apically fixed stent. In contrast, the annularly fixed are self-sealing after their deployment.
Since its first description in 2015, the concept of neo-LVOT after TMVR has become a concerning issue influencing the patient’s outcome11,13. Yoon et al retrospectively investigated 194 patients with TMVR for valve-in-valve, valve-in-ring, and valve-in-MAC procedures12. They simulated TMVR by multidetector row computed tomography and calculated the cross-sectional neo-LVOT area. Narrow neo-LVOT (below 1.7 cm2) was considered a predictive factor for LVOTO after TMVR. This occurred significantly more often in patients with calcified native mitral valves. Moreover, the data showed that patients with a neo-LVOT area ≤1.7 cm2 in preoperative CT assessment had an LVOTO rate of 66%, while the rate was 0.6% in patients with a neo-LVOT area >1.7 cm2. LVOTO was proven to be positively correlated with mortality after TMVR by the same study; the problem remains unsolved with currently used TMVR prostheses12.
The neo-LVOT is considered an extension of the anatomical LVOT in the left ventricle after TMVR which is composed of the base of the interventricular septum and the mitral prosthesis with or without the AML opposing the interventricular septum. Factors influencing the width of the neo-LVOT are prosthesis-related and anatomical. It is generally accepted that the shape and size of the ventricular part of the mitral prosthesis that forms the neo-LVOT are important prosthesis-related factors. Overheight and oversize may lead to the reduction in neo-LVOT dimensions. The thickness of the base of the interventricular septum is an anatomical factor that is negatively related to neo-LVOT size12. The problem of narrowing of the LVOT when leaving the native mitral valve in place during surgical mitral valve replacement has already been described by David et al19, and was addressed by Miki and associates. They developed a technique resolving the subvalvular apparatus, making a T-shaped incision on the anterior leaflet and re-suturing the two halves to the annulus near their respective commissures20. In TMVR, resection of the native valve is not possible. With respect to the innovative potential of TMVR, so far little is known about the neo-LVOT and its relevance after TMVR and the potential risk for post-interventional LVOTO.
The preoperative AMA, formed by the central axes of the mitral annulus and the LVOT, is a parameter that can significantly impact on the postoperative neo-LVOT dimensions. A smaller, more acute AMA may lead to lower risk of neo-LVOTO12. However, after TMVR, the atrioventricular canal is replaced by the tube-shaped stent-loaded valved prosthesis, and the AMA is formed by the central axes of the tubular structure and the LVOT. Obviously, the direction of the new central axis is similar to that of the native mitral annulus, but the shape and fixation method may alter the AMA. Hence, both prosthetic and non-prosthetic factors influence the AMA.
In both groups of our study, the AMA changed after TMVR compared with the baseline values. However, the maximal and minimal AMA were significantly larger after implantation in the ANN group. In the AP group, changes in AMA were not significant, and the postoperative AMA was even narrower. Obviously, such a difference is related to the transapical fixation system of the prostheses used in the AP group. As shown in Figure 4, because of the transapical fixation system, the central axes of stents in the AP group moved towards the apex of the left ventricle and led to a better stabilisation of the axes and to lower AMA. In the AP group, the postoperative changes in the AMA during the cardiac cycle (the difference between the maximal and the minimal angulations) were lower compared to preoperative changes. This indicated that the apical fixation system led not only to a smaller but also to a more stable AMA after TMVR.
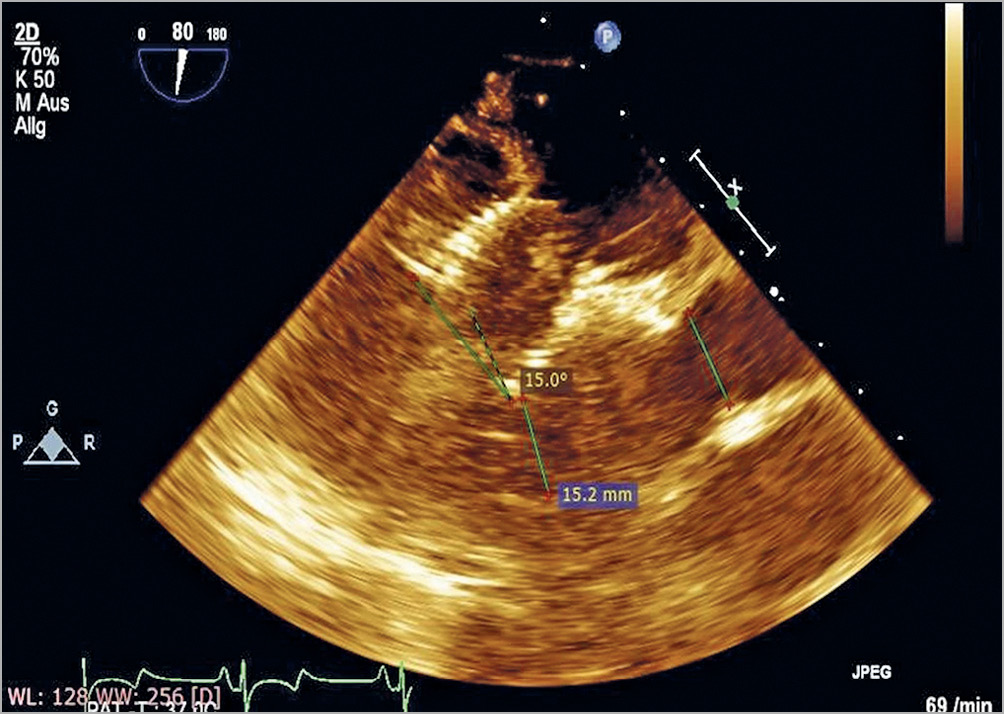
Figure 4. Left ventricular long-axis view after TMVR of the AP group. The central axis of the stent moved towards the left ventricle and led to a better stabilisation of the axis and to lower aorto-mitral angulation (AMA).
As we stated before, in the absence of an apical fixation system, the primary directions of the central axes of tubular implants and those of the baseline mitral annulus were similar, which was consistent with the designs of most current TMVR implants. However, the significant difference in changes of AMA showed the technical difficulty in maintaining a correct orientation of the central axis of implants. During the cardiac cycle, because of the prosthesis and its instability, ventricular systole and blood flow significantly disturb the AMA. During cardiac systole, the central axis of the implant changes with the mitral annulus and may sway in turbulence. If the implant is not stable, its displacement would be more evident than the movement of the native mitral annulus. Therefore, stable fixation of the implant leads to a lower change in central axis direction and the implant’s central axis is more consistent with the central axis of the autologous mitral annulus. Without annular fixation, blood flow during systole may push the interior part of the stent and displace the ventricular part of the stent towards the native LVOT, thus increasing the AMA significantly. Even by reducing the size of the membrane on the stent to reduce the impact area or resistance, the anterior leaflet of the mitral valve covers the exterior part of the stent and is subject to blood flow, bulging towards the LVOT. In some TMVR implants, the anterior leaflet may displace the implants. As shown in Figure 5, the AML was pushed towards the aortic annulus which might be the cause of increased AMA after TMVR. We believe that the apical fixation system not only stabilises the implants, makes the central axis apically oriented and reduces the AMA, but also reduces the impact of blood flow on the stent wall and further stabilises the implant during cardiac systole. In some clinical cases, the apical fixation can be done even more posterolaterally to reduce the AMA. This has not been done in this study. In the AP group all apical fixations were performed perpendicularly towards the true apex.
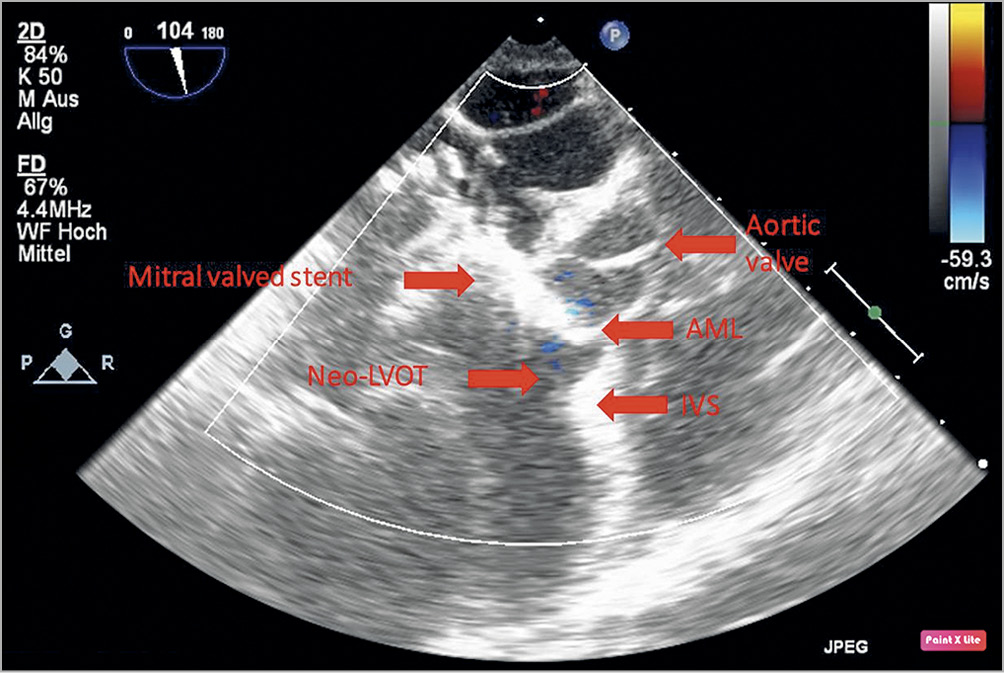
Figure 5. Left ventricle long-axis view after TMVR of the ANN group. The anterior mitral leaflet (AML) is pushed towards the aortic valve by the mitral valved stent and the blood flow causes systolic anterior motion (SAM) and an occluding neo-LVOT. IVS: interventricular septum
Aorto-mitral angulation was significantly associated with LVOTO in our experiments. Reduction in the width of the LVOT was over 60% in the ANN group and only 25% in the AP group. Peak LVOT velocity in the ANN group was nearly twice that in the AP group, and the narrowest width of LVOT was 12.9±8.3% of the baseline level at the end of the systolic phase. This may reflect the impact of blood flow on implant displacement.
Our study indicated that the AMA plays a critical role in the appearance of neo-LVOT after TMVR. The ANN group had a much narrower neo-LVOTd, even though the height of the ventricle body of the ANN group stents was shorter than that of the AP group stents. Perhaps the TMVR designers and developers should pay more attention to narrowing and maintaining the postoperative AMA to get an ideal area of neo-LVOT.
Furthermore, a tube-shaped ventricular element may not be the best option for the TMVR devices14. Firstly, it directly narrows the LVOT. Above all, it shortens the distance between the interventricular septal bulge and the prosthesis or the AML. Secondly, its inner wall behaves like a sail in the LVOT. If the ventricular body is too high, the stent together with the AML could be pushed towards the aortic annulus. Moreover, the stent wall blocks the closing motion of the AML. It may increase the risk of systolic anterior motion (SAM) of the AML after TMVR (Figure 6). It may be an option to cut off the part of the stent in the LVOT or fix the entire AML on the stent.
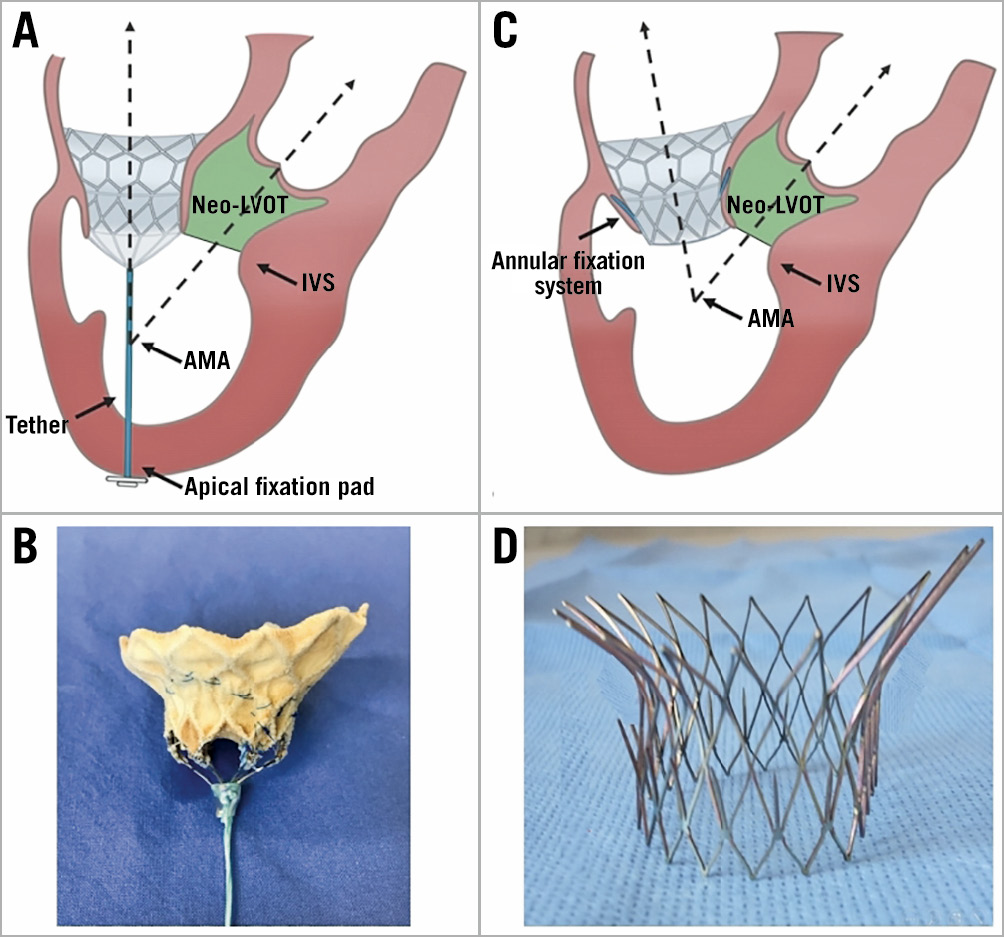
Figure 6. Schematic presentation: the influence between AP and ANN fixation on AML. A) In the AP group, the tether turned the stent towards the apical fixation pad, narrowed the aorto-mitral angulation (AMA) and enlarged the neo-LVOT. B) Implanted tethered D-shaped valve stent (AP group). C) In the ANN group, the stent faced towards the interventricular septum (IVS) and enlarged the AML. D) Implanted D-shaped annular fixed stent (ANN group).
Limitations
Though the study might have limitations due to the relatively small sample size, the large animal model offers the chance to create reproducible conditions that should be evaluated further in a clinical setting. Furthermore, the neo-LVOT is not a strictly simple circular structure and is dependent on many anatomical structures such as the interventricular septum, the AML and the atrioventricular prosthesis. The visualisation of the neo-LVOT would have been more precise by using additional 3D TEE in our experimental study. Nevertheless, for the AMA measurements and functional data, the 3D TEE would not have provided additional data.
Conclusions
In conclusion, our experiment demonstrates for the first time that an apical fixation system maintains a smaller and more stable AMA and a wider neo-LVOT, thereby reducing the risk of postoperative LVOTO after TMVR.
|
Impact on daily practice A narrowed neo-LVOT is associated with LVOTO after TMVR. Most TMVR prostheses are balloon-expandable annularly fixated and few focus on preventing the enlargement of the AMA which is a predictor of neo-LVOT. Our study demonstrated that the apical fixation system has a unique advantage in maintaining the AMA and reducing the narrowing of the neo-LVOT. |
Acknowledgements
We are very grateful to our animal facility managers, Drs Schultheiss and Vieten.
Funding
This project on TMVR is supported by the German DZHK (Deutsches Zentrum für Herz-Kreislauf-Forschung), Berlin, Germany.
Conflict of interest statement
D. Frank is a proctor for Edwards and Medtronic and has received travel grants from both companies. G. Lutter is a proctor for Medtronic and Edwards and has received travel grants from both companies. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

