Abstract
Aims: In this study two designs of a self-expanding valved stent were compared after off-pump implantation into the mitral valve to identify the superior one.
Methods and results: Two designs of a mitral valved stent were tested. The first design is composed of a circular atrial element connected to a tube-shaped ventricular element. In the second design, the atrial element is D-shaped to achieve better anatomical alignment. Prior to in vivo testing, the area with the highest risk of PVL was identified in a hydrostatic in vitro set-up. Subsequently, eight pigs received stents (circular, n=5; D-shaped, n=3) via apical access in the beating heart. Positioning and haemodynamics were evaluated by TEE and invasive pressure measurement pre-implantation, after 1 hr, and at two and four weeks. In vitro testing showed less PVL in the anteromedial region in D-shaped design stents (p<0.001). All stents were successfully deployed in vivo and six animals maintained normal haemodynamics for two weeks or longer. Rotational reorientation of all stents with D-shaped elements was observed. Both groups indicated no clinically relevant gradients over the mitral valved stent.
Conclusions: This study demonstrates that the circular design was superior to the D-shaped model after rotational reorientation of the latter occurred.
Introduction
Mitral regurgitation (MR) is one of the most common valvular heart diseases, its incidence increasing sharply with age1,2. As the Euro Heart Survey revealed, 49% of patients with severe symptomatic mitral regurgitation were non-compliant for open heart surgery3. Impaired left ventricular ejection fraction, older age and comorbidities were the most striking characteristics of these patients3.
In recent years, various techniques have been developed for percutaneous reconstruction of the mitral valve using an off-pump technique, most of them still being in the preclinical experimental phase. Another approach for percutaneous treatment of mitral valve regurgitation is the transapical implantation of a mitral valved stent using an off-pump technique. However, there are still no commercial stents available for clinical practice.
Off-pump implantations of aortic and pulmonary valved stents in humans have shown promising results4,5. Development of a valved stent for minimally invasive implantation into the mitral position is more challenging due to the complex anatomy of the mitral valve and its location in the high pressure system of the left heart. Previously, we have published different studies, demonstrating the feasibility of implanting mitral valved stents off-pump with follow-up periods of up to two months6-10. Those studies showed promising results, although different complications such as paravalvular leakages (PVL) and stent fractures were observed. Therefore, we continuously improved the stent design for better anatomical fit.
In this study, two different stent designs for replacement of the mitral valve are compared using an in vitro and in vivo model. The aim was to evaluate whether stents with a D-shaped atrial element are superior to stents with a circular atrial element with regard to positioning and preventing PVL as well as the development of stent fractures.
Material and methods
MITRAL VALVED STENT DESIGNS
Two designs of a self-expanding valved stent were tested: the first design was composed of a circular atrial element (Figure 1A, Figure 1B) connected at 45° to a tubular ventricular stent body (Figure 1C, Figure 1D). The nitinol stent frame (Euroflex GmbH, Pforzheim, Germany) was covered with an ultra-thin polytetrafluoroethylene membrane (Zeus Inc., Orangeburg, SC, USA) to minimise PVL. A tri-leaflet bovine pericardial or native porcine aortic heart valve was sewn into the ventricular body. A ventricular fixation system consisting of four individual neo-chordae was attached to the ventricular rim of the stent10.
For the second design, the aortal side of the circular atrial element was shortened, resulting in a D-shaped atrial element (Figure 1E, Figure 1F). The shortened edge of the D-shaped design was positioned towards the aortic root with the expectation of better anatomical alignment and hence a lower degree of PVL and fewer stent fractures.
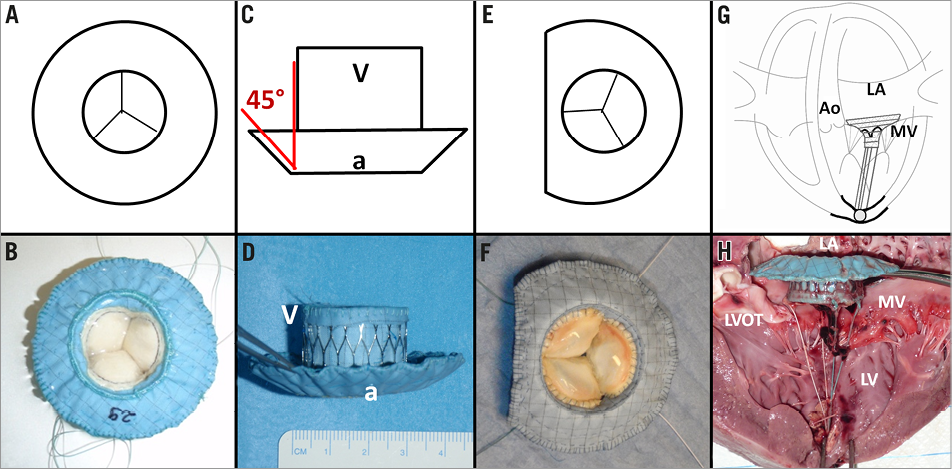
Figure 1. Images illustrating the different stent designs: A-B) Atrial view of the mitral valved stent with circular design; C-D) lateral view of the valved stent, red lines showing the angle (45°) in between the ventricular body (V) and the atrial element (a); E-F) atrial view of the valved stent with D-shaped design; G-H) lateral view of the mitral valved stent within the native mitral valve (MV) illustrating the ventricular fixation system which is composed of four neo-chordae. Ao: aorta; LA: left atrium
IN VITRO MODEL
Prior to implantation, both designs were evaluated in vitro in a hydrostatic test set-up (Figure 2). The central openings of the stents were sealed and the stents were placed in mitral position of defrosted porcine hearts (circular, n=4; average weight 430.5±98 g; D-shaped, n=4; average weight 373.3±24.6 g) and apically fixed with standardised tension, so that a shortening of the distance from mitral annulus plane to the apex was prevented by avoiding an invagination of the apex.
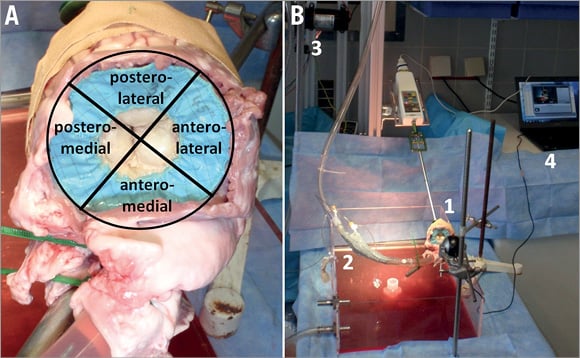
Figure 2. Images showing the in vitro test set-up. A) In order to evaluate the regional distribution of PVL, the mitral annulus was divided into four regions. B) Experimental hydrostatic test set-up with mitral valved stent in mitral position of a porcine heart: (1) test specimen, (2) catheter port, (3) Flojet pump, (4) computer for video acquisition.
The aorta was connected to a pump (Flojet membrane pump R4325-143; ITT Industries, White Plains, NY, USA) after resection of the aortic valve, and the left ventricular pressure was increased to 250 mmHg or until occurrence of PVL, which was visually monitored and rated. Following Carpentier’s segmental valve analysis11, the mitral annulus was divided into four regions: anterolateral, posterolateral, posteromedial and anteromedial. The location of PVL (regional distribution) under increasing left ventricular pressure and the pressure level at occurrence of mild PVL were recorded and evaluated. The experiment was repeated six consecutive times in each heart. PVL are indicated as a percentage of PVL observed in all regions during testing of the respective prototype.
IN VIVO MODEL
The experimental implantations were carried out using our well-established porcine model6-10. Eight pigs of the German Landrace and German Edelschwein with an average body weight of 48±2 kg underwent transapical off-pump mitral valved stent implantation. All animals received humane care, as approved by the Center for Experimental Animal Research at the University of Kiel, Germany, in compliance with the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources, National Research Council, and published by the National Academy Press, revised in 1996 and updated in 2011. Five animals received stents with circular and three animals received stents with D-shaped atrial elements.
IMPLANTATION OF THE VALVED STENTS
Total intravenous anaesthesia, continuous electrocardiographic monitoring and invasive measurement of blood pressures were conducted. The implantation of mitral valved stents was performed through a lower ministernotomy, which grants access to the apex of the heart in the porcine model. Two rows of 3-0 polypropylene pledgeted felt purse-string sutures were placed apically, and a heparin bolus of 150 IU/kg body weight was administered intravenously.
The valved nitinol stent was crimped into the delivery system, introduced through the apex of the heart and placed in position within the mitral annulus under transoesophageal echocardiography (TEE) guidance (Vivid i and probe 6T; GE Medical Systems, Waukesha, WI, USA). The tip of the delivery system was located in mid atrial position and the valved stent was deployed in a two-step procedure (Figure 3): first, the atrial element was deployed to allow positioning into the mitral annulus at the desired height. In a second step the ventricular element was released. After successful deployment, the delivery system was removed from the heart. During implantation of stents with a D-shaped atrial element, particular attention was paid to correct rotational orientation of the straight edge of the D-shape towards the aorta. The stent position within the mitral annulus and left atrium was adjusted under TEE guidance. Occurrence of PVL was monitored while adjusting the force on the ventricular fixation system. Correct positioning of the mitral valved stent was considered to be achieved when PVL was minimised. After correct positioning, the ventricular fixation system was knotted against a counter-bearing at the purse-string suture at the apex of the heart and the thorax was closed.
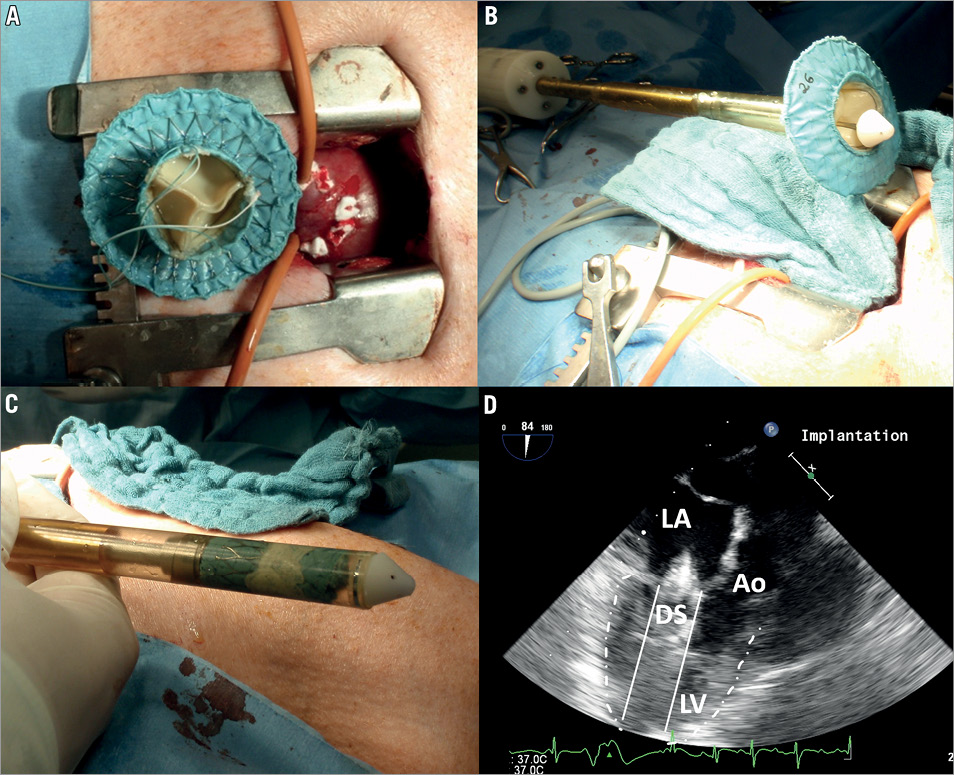
Figure 3. Images showing the mitral valved stent prior to implantation. A) Ventricular view of the mitral valved stent (circular design) and ministernotomy allowing apical access to the heart in the porcine model. B) Image of the mitral valved stent shortly before the crimping procedure. C) Mitral valved stent crimped within the proximal part of the deployment system. D) 2D TEE image of deployment system (DS) carrying a mitral valved stent with its tip placed in mid atrial position. Ao: aorta; LA: left atrium; LV: left ventricle
After successful implantation, the pigs were brought to the laboratory animal facility and monitored daily. Antibiotic therapy (enrofloxacin 5 mg/kg i.m., Baytril® 10%; Bayer AG, Leverkusen, Germany) was administered for ten days and general pain medication (carprofen 4 mg/kg i.m., Rimadyl®; Pfizer Inc., New York, NY, USA) for five days or as necessary. Animals did not receive any anticoagulation therapy.
MEASUREMENTS
Haemodynamic, electrocardiographic and echocardiographic data were recorded and analysed one hour and two weeks after implantation using central catheters, ECG and 2D TEE following a standardised protocol. TEE-based evaluation of the in vivo stent position, the occurrence of PVL and central mitral regurgitation was conducted in standard as well as non-standard views adapted to the pigs’ morphology.
If the condition of the animals was excellent, the observation period was prolonged and another TEE evaluation was conducted four weeks after implantation. A gross evaluation was performed after euthanasia of the animals.
STATISTICAL ANALYSIS
Statistical analysis was performed using SPSS 20 (IBM Deutschland, Ehningen, Germany). For all quantitative measurements, such as pressure values or deployment times, results were expressed as mean±standard deviation. In case of n≤2 single values are given. Based on the small number of observations, a test for normality will have very poor power. As no obvious outlying values were observed, normally distributed data were assumed and we used t-test statistics to compare groups. Counted data, such as leakages in different regions of the hearts in the in vitro experiments, were shown in frequencies and evaluated by the chi-square test. With regard to the small sample sizes, p-values were cited with an exploratory intention. The probability of a type I error was set to 5% (α=0.05).
Results
IN VITRO EXPERIMENTS
Analysis of the regional distribution of PVL showed a lower prevalence in the anteromedial region for D-shaped stents (12.5%) compared to stents with circular design (51.7%; p≤0.001). In the anterolateral region, changes between the groups of D-shaped (66.7%) and circular stents (34.5%) could be due to chance (Figure 4). PVLs occurred at higher pressures in the group of D-shaped design (130.9±59.2 mmHg) compared to the circular design (70.74±40.6 mmHg; p≤0.001).
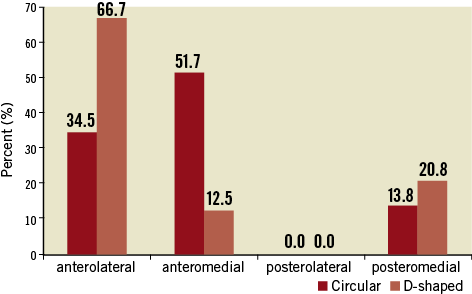
Figure 4. The regional distribution of PVL during the hydrostatic in vitro testing is indicated. The perceptual prevalence of PVL for each region is displayed for both prototypes.
IN VIVO EXPERIMENTS
All stents were successfully implanted in the beating heart. Deployment time for the circular stents was 162±28 s and significantly lower compared to the mean deployment time of 277± 85 s for the D-shaped stents (p=0.036). Six animals maintained normal haemodynamics with MAP and CVP constantly within physiological range. Two animals died after six hours due to their small hearts (n=1 in each study group). After ten days, the general condition of four animals was excellent, and the observation period was prolonged to four weeks (circular, n=3; D-shape, n=1). The remaining two animals were sacrificed after two weeks.
Echocardiographic evaluation revealed a good ejection fraction of 72±4% and 65±4% before and 66±4% and 54±4% after implantation for the circular group and the D-shape group, respectively. The mean gradients across the mitral valved stent and the aortic valve were low in both groups after implantation (Table 1). In the circular group, induration of the bioprosthetic stent valves was detected after one month (n=3). This finding corresponds to the increasing gradients and velocities over the mitral valved stent that were detected throughout the observation period, particularly in the circular group. However, due to low numeric changes throughout the observation period in the intra- and inter-group comparisons, this could be due to chance. The E/A ratios show a decrease, maximal velocities and velocity time integral an increase over time for both study groups (Table 1). However, no statistically significant variations were identified (p≥0.18).
Animals in the D-shaped group showed a higher degree of PVL in colour Doppler TEE in most cases (Table 2). PVL in this group were located near the shortened edge of the atrial element above the posteromedial commissure of the native mitral valve or anteromedial thereof. In the circular group, PVL was trace or less in three of four cases after two weeks. A moderate to severe PVL was detected in one case (case 2) in the anterolateral and at the posteromedial commissure. In one animal (case 5) mild PVL were detected on the anterolateral edge after one month.
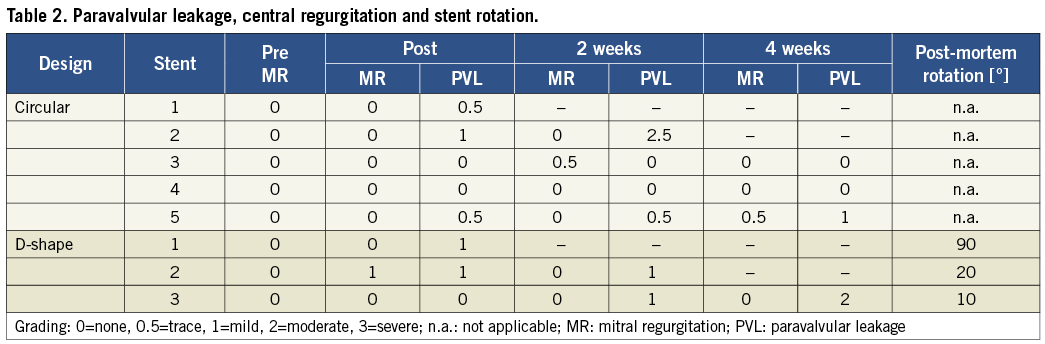
In addition, a rotational reorientation of D-shaped stents was observed. After confirmation of the correct positioning of the D-shaped valved stent in the postoperative TEE one hour after implantation, at later evaluation rotation of the stent was observed in all cases (Figure 5): a 10° posterior, a 20° anterior, and a 90° posterior rotation of the D-shaped stent were determined. These observations were confirmed in the corresponding post-mortem evaluations.
Figure 5. Schematic drawings and images showing the rotational orientation of the D-shaped stent after implantation. A-B) Schematic and TEE image of correct positioning of the D-shaped element towards the aorta (Ao) at implantation. C-D) Schematic and left atrial post-mortem view confirming the stent rotation (animal 1).
In-growths (Figure 6) and stent fractures of the mitral valved stent were evaluated in the post-mortem analysis (Table 3). Good in-growth of the valved stents was seen after one month, with 73±5.7% in the circular group and 97% in the D-shaped group. In one of three animals of this group the missing in-growths were located at the corners of the D-shaped side of the stent in the commissural area of the native valve.
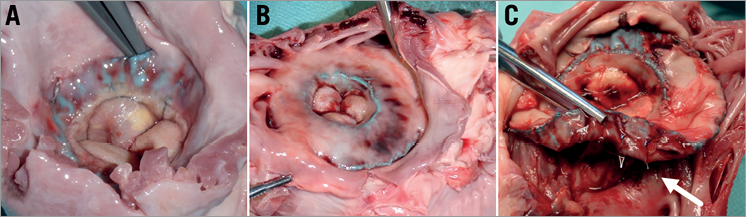
Figure 6. Images showing the in-growths as seen during gross evaluation. A-B) Gross examination revealed good in-growths after one month of the stents with D-shaped design (A) and circular stent design (B). C) Image of the mitral valved stent within the native anatomy during the gross evaluation. The white arrow indicates the stent fractures mainly observed in the junction between the atrial element and the ventricular body.
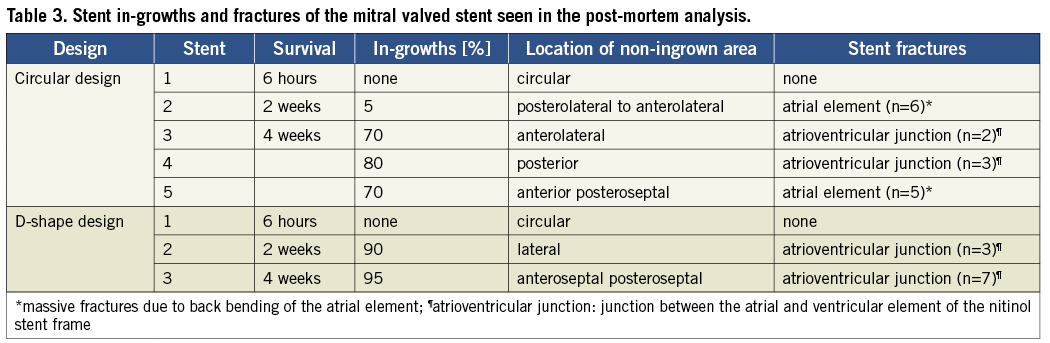
Stent fractures were found in both groups, mainly observed at the junction between the atrial element and ventricular body of the stent, and did not differ from each other. In two cases back bending of the atrial element in the anterolateral region led to massive fractures (Table 3).
Discussion
Minimally invasive mitral valve repair is the gold standard therapy of the diseased valve. However, this treatment carries a high surgical risk and approximately 30% of patients with severe symptomatic, single valvular heart disease are non-compliant due to advanced age or significant comorbidities2.
In recent years, transcatheter alternatives to conventional valve surgery have been developed, such as an implantation of valved stents in the beating heart, thus avoiding the use of cardiopulmonary bypass. These procedures have already been conducted in selected patients with aortic and pulmonary disease and have gained increasing acceptance for high-risk patients4,5.
The first experimental off-pump transcatheter stent implantation into the mitral valve using a transatrial approach was described by von Segesser and colleagues in 200512. In 2008, our group reported the results of our initial study concentrating on the technical feasibility of mitral valved stent implantation through a transapical approach in the porcine model6. In June 2012, the first-in-human transfemoral transcatheter mitral valved stent implantation was performed by Søndergaard and colleagues13. An 86-year-old male suffering from severe MR was treated and survived two days. Further details have not been published.
In the past, our group has reported on a multiple trial series demonstrating the feasibility of implanting mitral valved stents off-pump with follow-up periods of up to two months6-10. Those studies showed promising results such as good haemodynamics, correct stent positioning and minor LVOT obstruction. However, different challenges were identified in relation to PVL and stent fractures. Minimising PVL after stent implantation into the mitral valve is a key issue, as it is directly associated with the success of the surgery, influencing the patient`s life expectancy and quality of life14.
Therefore, continuous improvements in terms of better anatomical fit were conducted and two different stent designs developed and compared in this study: a circular and a D-shape design.
Correct positioning of the D-shape towards the aorta under TEE guidance was more difficult, as reflected by increased deployment times. Nevertheless, this was successfully achieved in all cases. However, a rotational reorientation was observed in all implanted D-shaped stents and secure positioning of the D-shaped edge towards the aorta was not achieved. Thus, the straight part of the D-shaped element was no longer positioned towards the aorta, but rather towards one of the commissures. Presumably this led to increased PVL, which were mainly observed in the region of the shortened edge of the D-shape element. Most likely the observed reorientation occurred during the first days, prior to the formation of stent-stabilising tissue bands between the stent and native structures of the heart. After having observed this rotational reorientation in three consecutive animals, the in vivo trial was abandoned. In the group with circular-shaped stents, PVLs were low in all but one case where moderate to severe PVL was detected after two weeks and the observation period was ended. PVL occurred at the posteromedial commissure, highlighting the challenge of adequate sealing in this area. Furthermore, gross examination showed massive stent fractures at the atrioventricular junction in the anterolateral region, where PVL had also been detected. Therefore, it is most likely that the augmented PVL were caused by fracturing of the nitinol frame.
The D-shape design tested in this study clearly did not provide sufficient rotational stability. However, it is likely that a relative rotational motion between the native mitral annulus and the valved stent also occurred in the circular group, but was not clinically observed due to its symmetrical shape. Additional anchoring mechanisms could prevent such a rotation and the intended advantages of the D-shaped design could then be achieved. Possible alternative fixation methods could include additional hooks or struts placed at the annular or subannular level. Such novel anchoring designs that fulfil the requirements of transcatheter implantation should be developed and analysed in further studies.
This stent behaviour was not observed throughout hydrostatic in vitro testing. On the contrary, the results of the latter supported the hypothesis of better anatomical fit of the D-shaped design, showing fewer PVL in the aortal region. In contrast to the in vitro model, the valved stent is anchored in vivo on a moving wall. The wall motion causes varying strength of pull on the ventricular fixation system, which is likely to influence the rotational behaviour of the valved stents. These findings highlight the importance of in vivo testing in addition to the in vitro testing. Even though in vitro experiments are of great importance, they imply various simplifications, hence omitting interaction of heart movement, contractility, electrical conduction and perfusion.
In vivo evaluation of haemodynamic parameters, such as ejection fraction and transvalvular gradients, showed no significant differences: only a tendency towards better results was seen in the circular group. This is most likely related to the higher degree of PVLs in the D-shaped group. However, gradients and velocities over the mitral valved stent increased throughout the observation period in the circular group, due to induration of the stent valves after one month.
In this study, no systolic anterior movement (SAM) of the native anterior leaflet and no thrombus formation were observed.
Gross evaluation of the mitral valved stent showed good in-growth of approximately 80% after one month, corresponding with the results of prior studies10. We do not believe that the differences between the two groups are related to the prototype designs, but rather to biological variation and other coherences observed in all our studies. We assume that, after complete in-growth of the valved stent into the surrounding anatomy, the stent structure plays only a secondary role supporting the valve and preventing PVL. The tissue growth between stent and native tissue might act as new annuli, substituting the dilated native mitral annulus in the clinical setting.
The healing response to the mitral valved stent is not yet clearly identified and is currently being analysed in a detailed histological and in-growth evaluation of a large cohort. Different studies on biological responses to other intracardiac devices, such as atrial septal defect occluders, show encouraging results with almost complete coverage of the devices by endothelial cells after three months15,16.
Gross evaluation additionally showed fracturing of the stents. No clear differences between the two groups were noted in this respect. Fracturing of the stent structure occurred mainly at the junction in between the atrial and ventricular elements. We consider the stent fractures mainly to be related to the overall fit of the stent within the native anatomy and to be caused by in vivo deformation of the stent resulting in areas of high stress. In future development the overall design of the mitral valved stent has to be optimised in order to improve the fatigue limit.
Study limitations
The long-term durability and fixation stability of these pericardial valved stents are unknown because of the relatively short-term nature of this study. Beating heart transcatheter mitral valved stent implantation was performed solely in healthy pigs. The effectiveness of the valved stents in eliminating mitral regurgitation in pathological hearts has to be proven. Furthermore, only pigs within a small range of body weights (45 to 51 kg) were used in this study, so the diameters of the native mitral annuli were within an adequate range. Therefore, stents of identical diameters were used. In all animals mild oversizing of the stents into the mitral annulus was observed. Prior to clinical implantation of a stented bioprosthesis into the mitral position, the diameter of the device must be adapted for the patient. In this study, a rotational reorientation of the D-shaped stents was observed. However, this reorientation also possibly occurred in the circular group. A future trial with marked circular stents to record this rotation could allow an indirect quantification of stent movement. The mitral valved stent with D-shaped atrial element was only implanted in a small group of three animals. However, a tendency of the results was found, specifically in relation to the lower number of PVLs resulting after implantation of a stent with circular atrial element design.
Conclusions
This study demonstrates the superiority of the circular design over the D-shaped model since rotational reorientation of the latter was observed. Consequently, a higher degree of PVL of all stents with a D-shaped atrial element was observed in vivo. Hence, an expected improved atrial alignment resulting in better sealing properties and fewer forces acting upon the stent was not achieved. However, preventing this reorientation of the D-shaped design by means of additional fixation or design change could make this possible. This should be investigated further.
Acknowledgements
We would like to thank Jürgen Hedderich and the Institute of Medical Statistics and Informatics for their excellent assistance as well as Florian Bönke, Holger Hettich, Jan-Paul Gundlach and Jawid Madjidyar for their valuable help throughout this study.
Funding
G. Lutter’s project on transapical mitral valve replacement is supported by the German Research Foundation, Bonn, Germany (Grant LU 663/8-1).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. 2-D TEE video showing the delivery system traversing the mitral valve into the mid atrial position.
Moving image 2. 2-D TEE video showing the mitral valved stent following implantation in three-chamber view. The shortened edge of the atrial element is positioned away from the aorta on the left side.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. 2-D TEE video showing the delivery system traversing the mitral valve into the mid atrial position.
Moving image 2. 2-D TEE video showing the mitral valved stent following implantation in three-chamber view. The shortened edge of the atrial element is positioned away from the aorta on the left side.

