
Abstract
Aims: The Mitra-Spacer (Cardiosolutions, Bridgewater, MA, USA) is designed to treat mitral regurgitation by introducing a dynamic spacer that constantly adapts to the changing haemodynamic conditions during the cardiac cycle. We aimed to evaluate the performance and safety of this device in the chronic ovine model.
Methods and results: Eight sheep were enrolled in this study. Through a left thoracotomy, the Mitra-Spacer was inserted via the transapical approach and advanced into the left atrium (LA) under imaging guidance. Device performance and safety were evaluated up to 90 days using fluoroscopy, echocardiography and histopathology. The volume within the balloon spacer shifted during the cardiac cycle in all cases. Seven animals survived up to 90 days for terminal imaging and tissue harvest. Echocardiography showed no change in left ventricle (LV) ejection fraction from baseline to 90 days. There were no observations of changes in LV diastolic function, pulmonary vein inflow, or tricuspid valve function. Histological analysis demonstrated no significant injury to the mitral apparatus.
Conclusions: In the healthy ovine model, Mitra-Spacer implantation was feasible and safe. At 90 days, no evidence of structural damage to the mitral apparatus or deterioration of cardiac performance was demonstrated.
Abbreviations
ACT: activated clotting time
AR: aortic regurgitation
EDV: end-diastolic volume
EF: ejection fraction
ESV: end-systolic volume
H&E: haematoxylin and eosin
LA: left atrium
LV: left ventricle
MR: mitral regurgitation
MV: mitral valve
TR: tricuspid regurgitation
Introduction
Mitral regurgitation (MR) is one of the most prevalent valve diseases and it is estimated to occur in almost 10% of people above 75 years of age1,2. While surgical treatment of MR is the gold standard of therapy, an important proportion of these patients is deemed to be too high risk for any surgical intervention due to associated comorbidities3-5. The progress achieved with percutaneous aortic valve technologies paved the way for the development of alternative therapies to treat high-risk patients with MR6,7. As these technologies continue to make clinical progress, it is expected that some could soon become a therapeutic alternative for this type of patient8,9.
The Mitra-Spacer™ (Cardiosolutions, Bridgewater, MA, USA) is a novel device designed to treat mitral regurgitation by introducing a dynamic spacer that constantly adapts to the changing haemodynamic conditions of the mitral apparatus and left atrium (LA). We present evidence of device safety and performance in a healthy sheep model, based on imaging data and histological results up to 90-day follow-up.
Methods
STUDY DESIGN
The study was conducted in accordance with the Guide for Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee (IACUC) of the CRF Skirball Center for Innovation. All animals received humane care in compliance with current guidelines. This study comprised chronic evaluation in a sheep model. The device was implanted in eight Polypay sheep (62.4±5.7 kg) via the transapical approach through left lateral thoracotomy under echocardiographic and fluoroscopic guidance. Post-deployment two-dimensional (2D) echocardiography, left ventriculography and fluoroscopic imaging were performed immediately following the implantation and at 30, 60 and 90 days following the procedure. All animals were maintained on warfarin therapy throughout the study. Seven animals were euthanised at day 90±5, and underwent a complete necropsy under the supervision of a board-certified veterinary pathologist; tissue was harvested and subsequently sent for histological examination.
PROCEDURAL DESCRIPTION
Oral antiplatelet therapy (325 mg aspirin, 150 mg clopidogrel) was administered one day prior to the procedure in all animals and continued daily for up to thirty days. Intravenous cefazolin (1 gram) was given 30 minutes prior to operation and every 90 minutes during the procedure, followed by 14 days of oral cephalexin (20 mg/kg twice a day). The animals were first anaesthetised with telazol (3 mg/kg IM) then placed on a mechanical ventilator and gas anaesthesia machine. General anaesthesia was maintained with isofluorane/oxygen. Following appropriate anaesthetic depth, a vascular access sheath was placed in the femoral artery using the Seldinger technique and a jugular vein was accessed to establish intravenous access for fluid and medication delivery, if necessary. A vascular sheath was introduced by cut-down.
Heparin was administered (100~200 U/kg IV), and bolus injections were repeated as needed (1,000-5,000 U IV) to maintain an activated clotting time (ACT) over 250 seconds. Animals were administered with subcutaneous lovenox (40 mg, one to two doses a day) for three to five days starting the day following the device implant. Oral warfarin therapy (dosage was determined by INR level) was initiated for each animal the day after device implant and was continued for the remainder of the study. Blood samples were collected at baseline, during and immediately after the implant procedure, and at interim (day 30±3) and termination (day 90±5) time points. On day 0 and at termination, blood samples were obtained as necessary during the procedures to monitor blood gas and electrolyte parameters during anaesthesia. Blood was drawn from the jugular vein or other peripheral vessel for haematology, coagulation, clinical chemistry, plasma free haemoglobin and/or fibrinogen; blood was also collected to monitor INR and/or ACT as frequently as necessary.
DEVICE DESCRIPTION
The Mitra-Spacer system is composed of a spacer balloon, shaft, heart pad and subcutaneous attachment tube (Figure 1). The Mitra-Spacer allows axial adjustment for neutral positioning of the device in the mitral valve (MV). The device is intended to be only partially inflated to allow the balloon to conform to the unique topographical variations found along the line of coaptation between the anterior and posterior leaflets specific to each MV. Partial filling of the spacer allows the ventricular portion to be filled during diastole when the MV is open, while during systole with increasing LV pressure the fluid inside the spacer moves towards the valvular level and the atrial portion, where the MV leaflets are pushed against the atraumatic surface of the Mitra-Spacer, thereby filling the MV defect and preventing regurgitation. The balloon is constructed from elastion, a biocompatible and biostable silicone polyurethane co-polymer. The attachment tube of the implant is fixed to the external surface of the left ventricle (LV) apex with a polyester-covered heart pad. Once the appropriate axial positioning is established, the heart pad fixes the position of the Mitra-Spacer in the MV. The attachment tube terminates where it connects to a subdermal inflation/deflation port. The port provides post-procedure adjustment of the device inflation volume for further optimisation of the Mitra-Spacer size if desired.
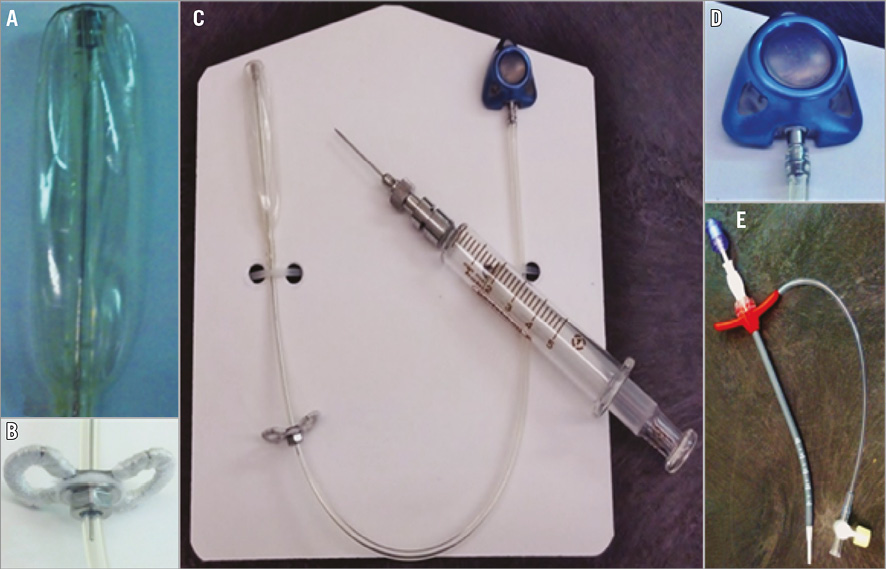
Figure 1. The Mitra-Spacer device and delivery system components. A) Deflated balloon spacer. B) Heart pad. C) Complete device view. D) Subdermal access port. E) 14 Fr tear-away introducer with valve.
DEVICE IMPLANTATION PROCEDURE
A left lateral thoracotomy was performed exposing the heart, and under fluoroscopy guidance the appropriate location was identified to make an apical puncture. Following heart exposure, the line between the potential puncture/access site and the centre of the MV annulus was assessed using 2D echocardiography. Purse-string sutures were placed around the marked apical access point using 3-0 or 2-0 Prolene sutures with Teflon pledgets. Then, the apex was punctured with an angiographic needle and the angle of the needle with the MV was confirmed by means of fluoroscopic and 2D echocardiography images. Next, a 0.018” atraumatic wire was inserted crossing the apical wall and into the LV. While the wire remained in place the needle was exchanged for an introducer. The introducer was advanced through the apex and into the heart so that the sheath penetrated inside the LV approximately 1 cm. The dilator and wire were removed and, with the sheath still in place, the prepared Mitra-Spacer was inserted into the sheath. The Mitra-Spacer was pushed through the sheath until approximately 1 cm was protruding out of the distal end. The Mitra-Spacer was then partially inflated, with the contrast mixture resulting in a “lollipop” appearance which aids in preventing entanglement with the chordae tendineae. The “lollipop” assembly was then advanced into the LA. The Mitra-Spacer was advanced until it was approximately 5±2 mm from the top of the LA. The sheath was retracted, completely exposing the Mitra-Spacer. The purse-string sutures were cinched as the sheath was removed from the heart. The device was inflated under guidance with an appropriate contrast mixture. The Mitra-Spacer was positioned such that approximately 50% resided in the LA and the other 50% in the LV. The sheath was removed from the Mitra-Spacer tubing. The heart pad was advanced against the apex and the nut was tightened. Finally, the inflation port location was determined in a subdermal location, leaving ample tubing for port placement. Through the port, with a non-coring needle, the spacer was aspirated to remove any air entrapped when connecting the port. As MR is artificially induced by the implant, adjustment to the amount of contrast material within the Mitra-Spacer was performed in order to reduce the regurgitation degree guided by ultrasound findings. Following the insertion of a chest tube, the thoracotomy incision was closed.
IMAGING EVALUATION
Imaging evaluation was performed before and after device implant and at 30, 60 and 90 days. 2D echocardiography and fluoroscopy were performed at all time points. Echo images were acquired in the right lateral decubitus position using a 5-MHz probe (iE33; Philips Medical Systems, Bothell, WA, USA) from standard parasternal long- and short-axis planes. LV end-diastolic diameter and end-systolic diameter were determined from short-axis planes; end-diastolic volume (EDV) and end-systolic volume (ESV) were calculated using Simpson’s rule, and LV ejection fraction (EF) was calculated using a standard formula (EF=[(EDV-ESV)/EDV] ×100). The degree of procedure-induced regurgitation was assessed as well as tricuspid regurgitation, aortic regurgitation, MV E (cm/s), MV A (cm/s), E:A ratio, balloon diameter (mm), and MV diameter (mm). Cardiac output was measured at baseline and termination time points.
Fluoroscopic evaluation at baseline and termination included qualitative assessment of LV function. All images were analysed by experienced imaging technicians.
HISTOLOGICAL EVALUATION
All animals that were euthanised at scheduled termination or early death were subjected to a full necropsy by trained staff under the supervision of a board-certified veterinary pathologist. A necropsy consisted of the removal of the heart and gross visual assessment of the body surfaces, cavities and their contents, which included the heart, lungs, kidneys, liver, spleen, and brain, which were sampled and immersion fixed in 10% neutral buffered formalin for histological processing and evaluation. MV leaflets, including annulus and adjacent myocardium, were sectioned along the long axis (i.e., longitudinal) to yield four approximately evenly spaced planes from commissure to commissure. Each trimmed leaflet section was paraffin processed, embedded, and stained with haematoxylin and eosin (H&E) and Movat’s pentachrome or elastic trichrome. The ventricular myocardium, from apex to base, was serially sectioned every 5 mm to assess for the presence of macroscopic changes. Histological examination included a sample from the mid and base portions of the anterior, lateral and posterior walls of the left and right ventricles. All myocardial sections were embedded in paraffin and stained with H&E. Similarly, sections of the left atrium were examined. Histological scoring via light microscopy was used to grade parameters that reflected the degree and extent of the overall host response to the implanted device and/or the presence of adverse pathology in the heart or other tissues, including inflammation, presence of thrombus, degree of endothelial cell loss, haemorrhage, and fibrosis.
STATISTICAL ANALYSIS
All information was collected and processed in an Excel database (Microsoft Corporation, Redmond, WA, USA). The following values were calculated: average (mean), standard deviation and percentage (whenever applicable).
Results
PROCEDURAL RESULTS AND IMAGING FINDINGS
A total of eight sheep underwent device implantation (Figure 2) without any periprocedural complications. Postoperative recovery was normal in all animals and no adverse effects were seen immediately following device implantation. The mean total procedural time was 154±21 minutes. One sheep died seven days following device implantation: autopsy findings revealed evidence of severe gastrointestinal bleeding, presumably resulting from chronic anticoagulation therapy. Seven animals survived the entire 90±5 days survival period. No other animals experienced any device-related postoperative complications.
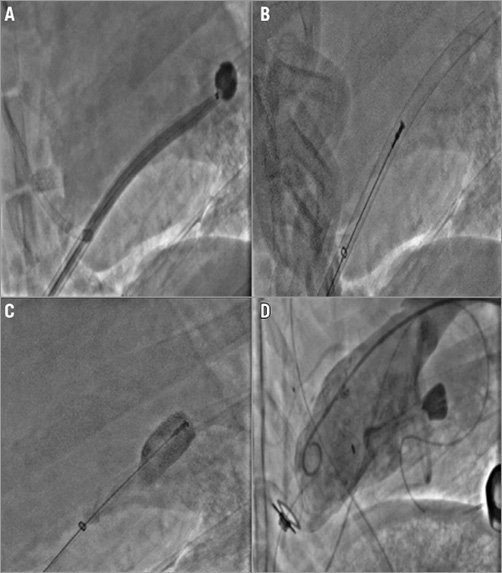
Figure 2. Step-by-step implantation procedure. A) The messenger balloon is delivered through the introducer and advanced to the LA clear of the chordae tendineae, followed by advancement of the introducer to LA. B) Once in the LA, the messenger balloon is removed and Mitra-Spacer introduced. C) The introducer is then removed and the Mitra-Spacer is filled with iopromide/saline mix. D) Final fluoroscopic images of implanted Mitra-Spacer during LV ventriculography.
Angiographic analysis of the LV following device deployment demonstrated that all devices were properly positioned with 64±9% of the device lying below the mitral annulus and 36±9% in the LA (Figure 3). At termination, it was observed that approximately ~60% of the mitral spacer still resided in the LV and ~40% resided in the LA. In the angiographic analysis, three sheep presented hypokinesis of the apex, one immediately post device implantation and two at termination.
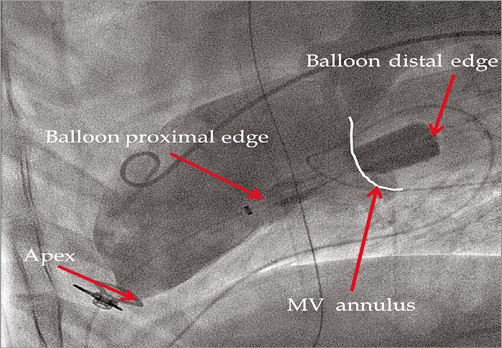
Figure 3. Fluoroscopic image of fully deployed device with arrows pointing to heart structure and device components.
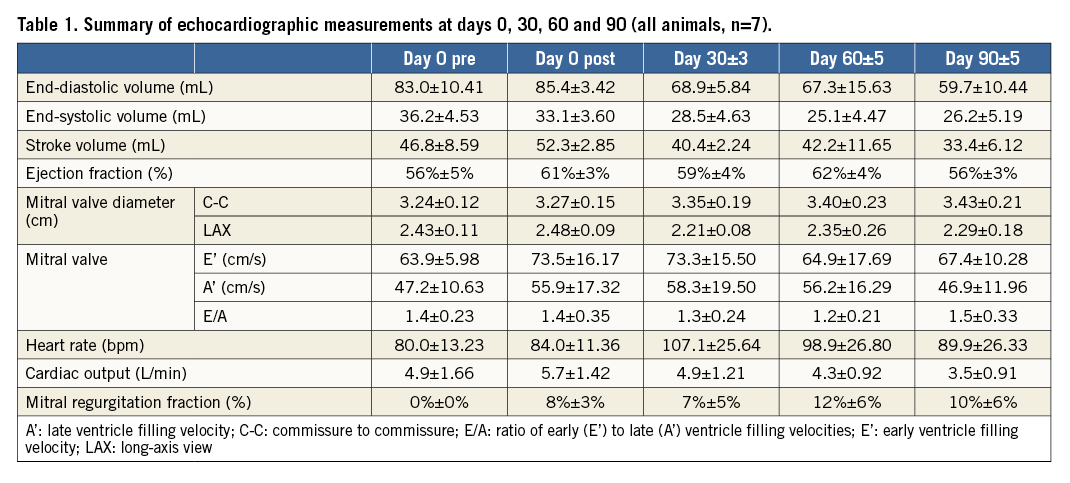
Echocardiographic evaluation revealed that values for end-diastolic volume, end-systolic volume, stroke volume, and cardiac output slightly decreased over time as compared to baseline values. However, LV ejection fraction remained unchanged over time. In addition, the percentage of mitral regurgitation fraction increased slightly over time (12±6% at 60 days and 10±6% at 90 days) (Table 1). There were no cases of either secondary aortic regurgitation (AR) or tricuspid regurgitation (TR) in any of the animals at follow-up.
DEVICE-HEART INTERACTION: MACROSCOPIC FINDINGS
At termination, all animals surviving to the protocol-defined endpoint (day 90±5) showed no evidence of injury to the heart or any of the greater vessels. Heart dissection showed that all devices were securely implanted in the LV apex of all sheep. In gross pathology, the three sheep that displayed angiographic evidence of apical hypokinesis did not present any gross abnormalities either in the LV or in the MV. Presence of LV endocardial fibrosis at the apical region was observed as a consequence of the healing response to the access site. There was no evidence of device-related infection. Both native mitral leaflets were spared without any evidence of rupture, thrombosis or infection. The subvalvular apparatus was spared and had a normal appearance in all studied animals. The left atrial endocardial surfaces were free from device-related lesions, including thrombus deposits (Figure 4).
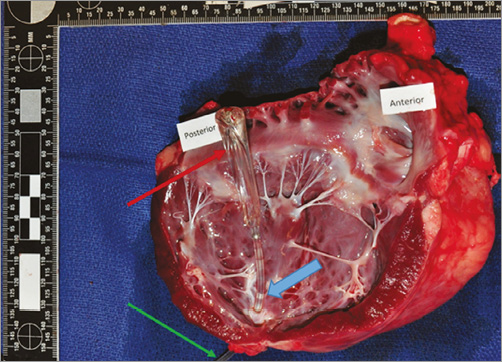
Figure 4. Image of heart necropsy showing intact balloon spacer (red arrow) next to the anterior leaflet of the mitral valve (anterior) and the posterior leaflet of the valve (posterior). The shaft of the device is incorporated into the left ventricle apex (blue arrow) and a small part of the attachment tube is seen emerging from the heart (green arrow).
On the surface of the device, organised thrombi (mean length 13.3±5.6 mm) were present along the apical aspect of the device shaft in three out of seven sheep. Thrombus formation was not seen at the luminal endocardial surface of the heart in any of the implanted animals at 90 days. Also, in four animals, layered thrombus material (mean length 3±2.4 mm) was present at the distal aspect of the spacer balloons.
SYSTEMIC EMBOLISATION EVALUATION
At termination, evaluation of all peripheral organs revealed no macroscopic abnormalities that could be associated with the delivery or presence of the device. One sheep was found to have bilateral kidney infarction while four others had unilateral small infarcts. Evidence of distal embolisation was not seen in any of the other peripheral organs or peripheral vessels examined. Organised thrombi were seen in the diaphragmatic lung lobes of two sheep: these thrombi were thought to originate from the venous indwelling catheter (used for daily monitoring of INR values) and were not considered to be device-related. Evidence of distal embolisation was not seen in any of the other peripheral organs or vessels including liver, spleen, oesophagus, stomach, small and large intestines, pancreas, brain, abdominal aorta, mesenteric vessels, iliac arteries or lungs.
DEVICE-HEART INTERACTION: HISTOLOGICAL FINDINGS
In the histopathology analysis, no evidence of device-related complications including perforation, laceration, necrosis, exuberant inflammation or endocardial changes was noted. The mitral leaflets and subvalvular apparatus were spared without any evidence of device-related injury. The mitral leaflets on the atrial side displayed lower endothelial coverage (mean endothelial loss score 1.7±0.72) and increase in endocardial hyperplasia (mean hyperplasia score 0.47±0.18) compared to the ventricular side of the leaflets (mean endothelial loss score 0.7±0.32, mean hyperplasia score 0.04±0.07), but without evidence of thrombus formation. Endocardial hypertrophy and fibrosis of the leaflets were both minimal though more pronounced on the atrial leaflet side (Figure 5). The primary cellular infiltrate observed in sections of the MV and the myocardium was of lymphohistiocytic cells. To a much lesser extent, similar infiltrate was observed in the atrial side of the leaflets and atrial myocardium. Overall, the device-related histological changes of the MV were interpreted as minimal and biologically insignificant (Figure 6). The overall tissue response to the heart pad was confined to the device/tissue interface with no extension into adjacent myocardium. The predominant tissue response to the anchor was mild inflammatory infiltrate composed of histiocytes, giant cells and lymphocytes, which was limited by a thin outer fibrous capsule. The surrounding wall of the myocardium displayed some degree of fibrosis and inflammation and infrequently formation of minimal to mild fibrin-rich thrombus material, generally extending from the level of the heart pad to the endocardial surface and along the spacer shaft. In the lungs of two animals, pulmonary arteries were occluded by chronic thrombus with evidence for bacterial colonies. In the kidneys of those animals, there was evidence for subchronic cortical renal infarcts. In an additional three animals, chronic cortical renal infarcts were seen, classified as minimal in two of them and marked in the remaining animal.
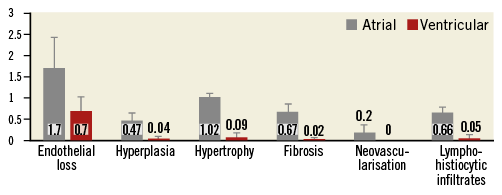
Figure 5. Comparison of histologic findings between atrial and ventricular leaflet sides. Endothelialisation findings are displayed according to the following scoring matrix: 0=no loss of endothelium; 1=<25% loss of endothelium; 2=25% to 50% loss of endothelium; 3=51% to 75% loss of endothelium; 4=>75% loss of endothelium. All other parameter findings are displayed according to the following scoring method: 0=no observable change; 1=minimal feature/presence in the tissue; 2=mild feature/presence in the tissue; 3=moderate feature/presence in the tissue; 4=severe feature/presence in the tissue.
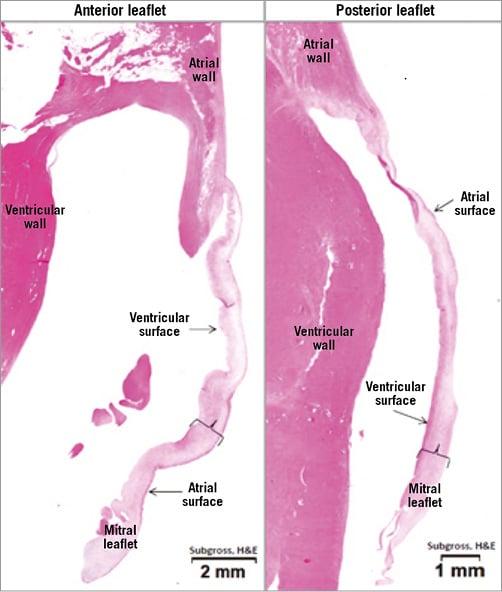
Figure 6. Representative image of cross-sections of the mitral leaflets stained with haematoxylin and eosin, showing relatively normal appearance of mitral valve leaflets with only slightly altered valve morphology.
Discussion
Symptomatic severe MR is an indication for valve surgery1, but many such patients are high surgical risk due to comorbidities5, resulting in poor prognosis due to heart failure complications5,10,11. During the last decade, the interventional field has witnessed a surge in the development of catheter-based technologies designed to treat this condition7. At this early stage of development, these interventions aim to target the subset of patients deemed to be inoperable.
The anatomical heterogeneity seen in MR patients is one of the major challenges in the development of catheter-based mitral intervention therapies10. As a consequence, a wide variety of transcatheter repair and replacement approaches has been developed and these are already under clinical investigation7. While most of these technologies target correcting the anatomical abnormalities seen in MR patients, other technological approaches aim to improve the abnormal haemodynamic profile occurring in this condition. In this study, we tested the long-term performance and safety of a novel device (Mitra-Spacer), designed to reduce MR independently of the valve morphology by self-adjusting to the dynamic changes seen during the cardiac cycle.
The Mitra-Spacer is a transapically delivered balloon designed for dynamic sealing of the regurgitant mitral area while being anchored to the LV apex. The device design is characterised by its dynamic ability to change dimensions throughout the cardiac cycle, and also to adjust the degree of sealing needed via the subdermally placed tube connected to the intracardiac balloon. The device is designed to adapt to the changing anatomy while minimally affecting the native MV function. It has been proposed that the apical anchoring mechanism used in this device plays an important role as it also integrates myocardial performance in its mechanism of function.
In this study, we evaluated the long-term safety of the device using the ovine model in which all devices were successfully implanted without any complications. Following device implantation, all animals survived without any evidence of haemodynamic compromise. No device-related clinical complications occurred during the device insertion procedure or at any of the follow-up time points. At 90 days, all valve components were preserved without evidence of damage to the leaflets or the chordae. No clinical signs of heart failure were observed. This was supported by preserved cardiac function with 2D echocardiography and fluoroscopic analysis. However, all animals developed a slight degree of MR and decrease in myocardial performance, which was expected since healthy animals were exposed to changing haemodynamic and mechanical conditions induced by the device. The device shaft was anchored to the left apex and the spacer location remained stable, both in their respective designated locations throughout the study. Finally, there was no macroscopic evidence of infection associated with the device or subcutaneous/transthoracic port/tubing and there were no major systemic thrombotic events in the brain, liver, lungs, spleen or gastrointestinal tract.
An important aspect of the study was the evaluation of device-related thrombogenicity and the potential for systemic embolisation. At 90 days, a small amount of layered thrombus adhering to the shaft at the apical insertion site was seen in three out of seven animals. The amount of thrombus formation found was very small and in a very advanced form of organisation. In addition, small chronic renal infarcts were observed in five out of seven sheep. It was concluded from the pathology evaluation that these renal infarcts might have been the result of the acute haemodynamic changes occurring at the time of device insertion and were unlikely to be embolic in nature. Additional non-device-related thrombotic events were also seen (i.e., chronic pulmonary septic thrombosis in two cases), suggesting a possible relationship to procedure- or model-related factors as the cause of these pathological findings. In this study, all animals were treated for one month with both dual antiplatelet therapy (i.e., clopidogrel and aspirin) and warfarin, followed by two months of warfarin only. Due to the unpredictable response of the ovine model to common anticoagulation strategies, we believe that some of these pathological findings may be the result of either: a) periprocedural factors, b) the presence of indwelling catheters, or c) suboptimal anticoagulation, and may have no clinical implications in humans. It is important to note, however, that experimental models do not provide the ideal environment for the evaluation of device thrombogenicity, and the real potential for device-related thrombotic risk of this device needs to be studied prospectively in human clinical studies.
In summary, in the ovine model, the Mitra-Spacer transapically delivered balloon spacer was both feasible and safe up to 90 days. Our data support the experimental reports with an earlier-generation device as well as a case report of one short duration attempt in humans12. This report showed that the mitral valve spacer was well tolerated, reduced MR and improved LV function. It was proposed in this human case report that the device had the combined effect of not only treating MR but also improving LV performance in a symptomatic patient12. If properly validated in clinical trials, this technology has the potential to be used in very high-risk MR patients using minimally invasive catheter-based techniques without having significant perioperative or long-term complications13,14.
Limitations
The sheep model is used extensively for the evaluation of therapeutic valve technologies despite its inherent anatomical and haemodynamic differences. However, it provides an acceptable anatomical and physiological environment for the evaluation of the device tested in this study. Also, the study was performed in healthy animals without the presence of MR, hence we were unable to test the impact of the device on efficacy in the presence of a dilated annulus and severe congestive heart failure. Additionally, in the ovine model, the therapeutic effect of warfarin is unpredictable and difficult to monitor due to the complex digestive system and pronounced influence of diet on drug absorption. As a result, sheep need a much higher dosage of warfarin compared to humans, displaying a wide therapeutic variability between animals15,16. Finally, the chronic use of indwelling jugular catheters for blood collection could be a source of the microembolisation seen in some of the animals, making it difficult to assess the origin of microemboli found in remote organs.
Conclusions
In the healthy ovine model, Mitra-Spacer implantation was feasible and safe. At 90 days, no evidence of structural damage to the mitral apparatus or deterioration of cardiac performance was demonstrated.
| Impact on daily practice The Mitra-Spacer is a novel device, which could provide clinical improvement for mitral regurgitation patients. This device could be implanted in high surgical risk patients. Its delivery and implantation are deemed safe and feasible in the current report in an animal model. |
Funding
This study was sponsored by Cardiosolutions Inc., West Bridgewater, MA, USA.
Conflict of interest statement
J. Wilson was Chief Operating Officer of Cardiosolutions Inc. C. Seguin was employed by Cardiosolutions Inc. during the study period. The other authors have no conflicts of interest to declare.

