Mitral regurgitation (MR) is a complex disease with multiple aetiologies and a high overlap with symptomatic heart failure. In terms of valvular heart disease, mitral regurgitation is the most common condition, with its prevalence increasing along with population ageing and increasing rates of heart failure1,2. An estimated two million adults in the USA have moderate to severe MR3,4. While surgery remains the “gold standard” treatment for significant MR, the majority of patients treated with surgery suffer from primary MR, rather than secondary MR. Most patients with severe secondary MR are diagnosed with symptomatic heart failure and reduced ejection fraction5.
Surgical treatment is often delayed or deferred in a large number of patients suffering from either primary or secondary MR4,6. The EuroHeart Survey revealed that up to 50% of symptomatic patients hospitalised with severe MR are not referred for surgery - mainly due to advanced age, comorbidities, and impaired left ventricular function which makes surgical risk unacceptably high. A study from the University of Michigan demonstrated that, while 59% of patients with primary MR underwent surgical repair or replacement, only 15% of patients with severe mitral regurgitation due to secondary MR underwent surgery7. In patients aged 80 years or more, surgical treatment was performed in only 15%, as compared to 60% in patients aged 70 years and younger8.
Although minimally invasive mitral valve surgery (MIMVS) has become a well-established and increasingly used option to treat patients with significant MR, the demand for alternative treatment options which are less invasive and avoid the need for cardiopulmonary bypass is rapidly growing due to the increase in rates of patients with advanced age and heart failure3,8. In the USA alone, the number of people aged 80 years or more is expected to increase from 6.9 million (2.8% of the population) in1990 to more than 25 million (5.7%) by the year 20509. Data from the Society of Thoracic Surgeons (STS) database indicate that the risk of mitral valve surgery increases markedly with age and, in the subgroup of patients aged 80 years or more, mortality and morbidity rates of 17.0% and 35.5%, respectively, have been reported10. On the other hand, it is also known that medically treated patients with severe symptomatic MR have 50% mortality within five years11.
Recently, various novel percutaneous transcatheter valvular technologies have emerged as possible alternatives to open surgery for high-risk patients12. Without the necessity for open heart surgery and cardiopulmonary bypass, these percutaneous procedures may be of particular interest to multimorbid and frail patients.
Medtronic’s transcatheter mitral valve (TMV) replacement device was presented for the first time at EuroPCR 2013, Paris, France (Figure 1). The self-expanding nitinol frame features a large inflow atrial portion that is responsible for sealing, and a short outflow ventricular portion designed to avoid LVOT obstruction. The nitinol characteristics of the inflow section of the frame are decoupled from the outflow section in order to optimise the conformability of the device to the individual patient’s annulus, while maintaining the structure of the outflow section to optimise haemodynamics and durability of the trileaflet pericardial valve. The bioprosthesis does not rely on outward radial forces for anchoring; instead, it uses the mitral apparatus for axial fixation (Figure 2) via capture of the anterior and posterior leaflets of the native mitral valve by support arms attached to the outflow section of the frame. Leaflet capture also minimises the risk of systolic anterior motion (SAM).
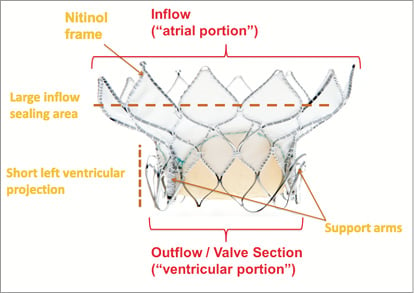
Figure 1. Medtronic transcatheter mitral replacement valve. The valve consists of a self-expanding nitinol frame, featuring a large inflow atrial portion and a short, circular outflow/valve section. Support arms are used to capture the native mitral leaflets at the A2-P2 position.
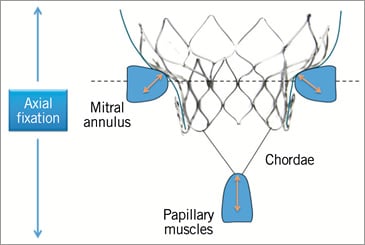
Figure 2. Medtronic transcatheter mitral replacement valve. The bioprosthesis does not rely on outward radial forces for anchoring, but instead uses the mitral apparatus for axial fixation.
The current device is delivered via a transatrial approach, using a procedure similar to a minimally invasive surgical mitral valve repair. The minimally invasive transatrial approach is intended to avoid inducement of further akinesis in left ventricular wall function, which is a possible risk with a transapical approach, as well as to avoid potential complications associated with the thin and friable tissue of the left ventricular wall common among secondary MR patients with severely enlarged ventricles. A transseptal delivery system is under development13. 2D and 3D echocardiographic imaging in combination with fluoroscopy are used to guide delivery of the implant, as well as to verify implant final positioning and valve performance.
To deploy the implant, the delivery system is inserted into the left atrium, typically at Waterston’s groove, and advanced across the mitral valve into the left ventricle. The transatrial delivery system is designed with a steerable tip to ensure coaxial positioning across the valve and to allow a controlled and accurate deployment. There is no need for rapid pacing during the deployment phase. Furthermore, acute animal experiments have not suggested compromised blood flow by virtue of stable haemodynamics during valve deployment. The orientation of the support arms is verified via fluoroscopic imaging to avoid entanglement with the tendinous chords of the mitral apparatus, and then the support arms are unsheathed at the level of the papillary muscle heads (Figure 3). The delivery catheter is retracted to allow the support arms to capture the anterior and posterior leaflets of the mitral valve at the A2-P2 position. Should the operator experience difficulty capturing the leaflets or is dissatisfied with the positioning of the support arms, the delivery catheter can be re-advanced into the left ventricle and the support arms can be fully recaptured and then redeployed to allow multiple attempts at leaflet capture. Once the operator is satisfied with leaflet capture, the valve section of the implant is unsheathed, followed by the inflow section (Figure 4, Figure 5). Once the inflow section of the implant is fully deployed, positioning and performance of the valve are verified via both 2D and 3D echocardiographic imaging, as well as fluoroscopy. Using a “bail-out” catheter, the implant can be fully retrieved and recaptured after being fully deployed, if not satisfied with implant positioning or valve performance.
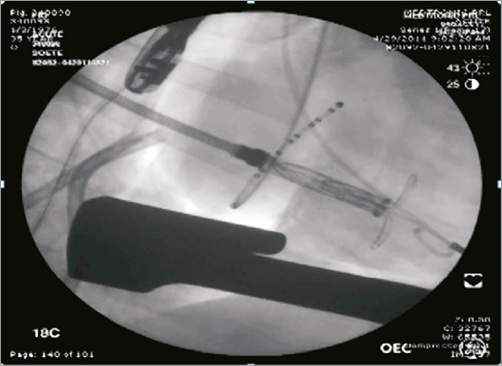
Figure 3. Medtronic transcatheter mitral replacement valve. Support arms are unsheathed in the left ventricle at the level of the papillary muscle heads.
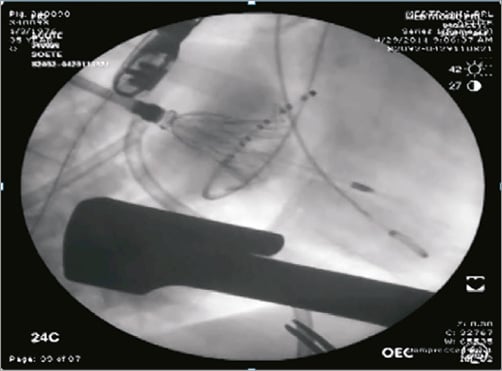
Figure 4. Medtronic transcatheter mitral replacement valve. Once the leaflets are captured by the support arms at A2-P2, the valve section of the bioprosthesis is unsheathed.
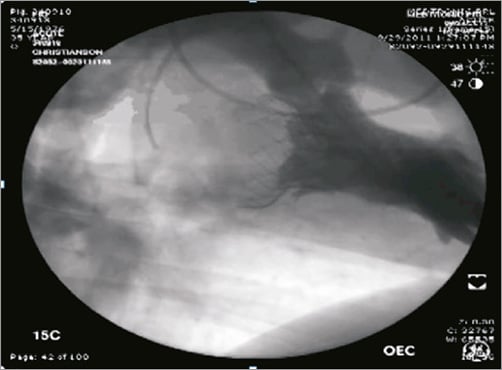
Figure 5. Medtronic transcatheter mitral replacement valve. The large inflow atrial section of the valve provides sealing area to minimise the risk of paravalvular leak.
In acute animal studies, Medtronic’s TMV prosthesis has been successfully implanted with an accurate and stable positioning. Importantly, there was absence of significant central or paravalvular leakage, LVOT obstruction and transvalvular gradients in the vast majority of experiments (Figure 5). No tearing or damage of the mitral leaflets or mitral apparatus has been observed following the recapture of the device. Chronic animal studies have been completed, and additional studies are currently ongoing to verify long-term device performance. With increasing preclinical knowledge, patient selection criteria and first-in-human studies will be addressed.
Conflict of interest statement
N. Piazza has received research grants from Medtronic and St. Jude, and is a proctor, consultant and scientific advisory board member for Medtronic, consultant/shares with Highlife, consultant/shares with CardiaQ. S. Bolling is a consultant and scientific advisory board member for Medtronic, Abbott and Edwards Lifesciences. N. Moat is a proctor, consultant and scientific advisory board member for Medtronic. H. Treede is a proctor and scientific advisory board member for Medtronic.

