
Abstract
Given the complexity and the heterogeneity of mitral valve anatomy and pathology, different technologies and approaches (including repair and replacement methods) are potentially required to allow specific patient-tailored approaches, addressing each anatomy with the most appropriate device. Since we are still far from having an unbiased and evidence-supported process to select the best treatment for each patient, this review will provide an overview of the elements that should be taken into consideration when selecting the best procedure for each patient.
Introduction
Transcatheter mitral valve therapies have the great potential to address unmet clinical needs for patients with symptomatic severe mitral regurgitation (MR) who are inoperable or at high surgical risk. Different approaches, including both valve repair and replacement, have been reported and are currently available or under investigation1,2. So far, transcatheter repair has been performed in tens of thousands of patients, while patients treated with transcatheter mitral valve replacement (TMVR) number about 350-400, including all the different devices.
A PATIENT-TAILORED APPROACH: THE IMPORTANCE OF PATIENT SELECTION
Given the complexity and the heterogeneity of mitral valve anatomy and pathology, different technologies and approaches are potentially required to allow specific patient-tailored approaches, addressing each anatomy with the most appropriate device. On the other hand, we are still far from having an unbiased and evidence-supported process to select the best treatment for each patient, and direct comparisons between transcatheter repair and replacement methods are not available so far.
The general consensus is that, in the near future, different types of interventional approach including mitral repair and replacement will be complementary rather than mutually exclusive. This will happen for both degenerative (primary) and functional (secondary) MR treatment.
Figure 1 shows a simple algorithm to select the possible therapeutic strategy in both degenerative mitral regurgitation (DMR) and functional mitral regurgitation (FMR) patients according to the clinical and anatomical profile.
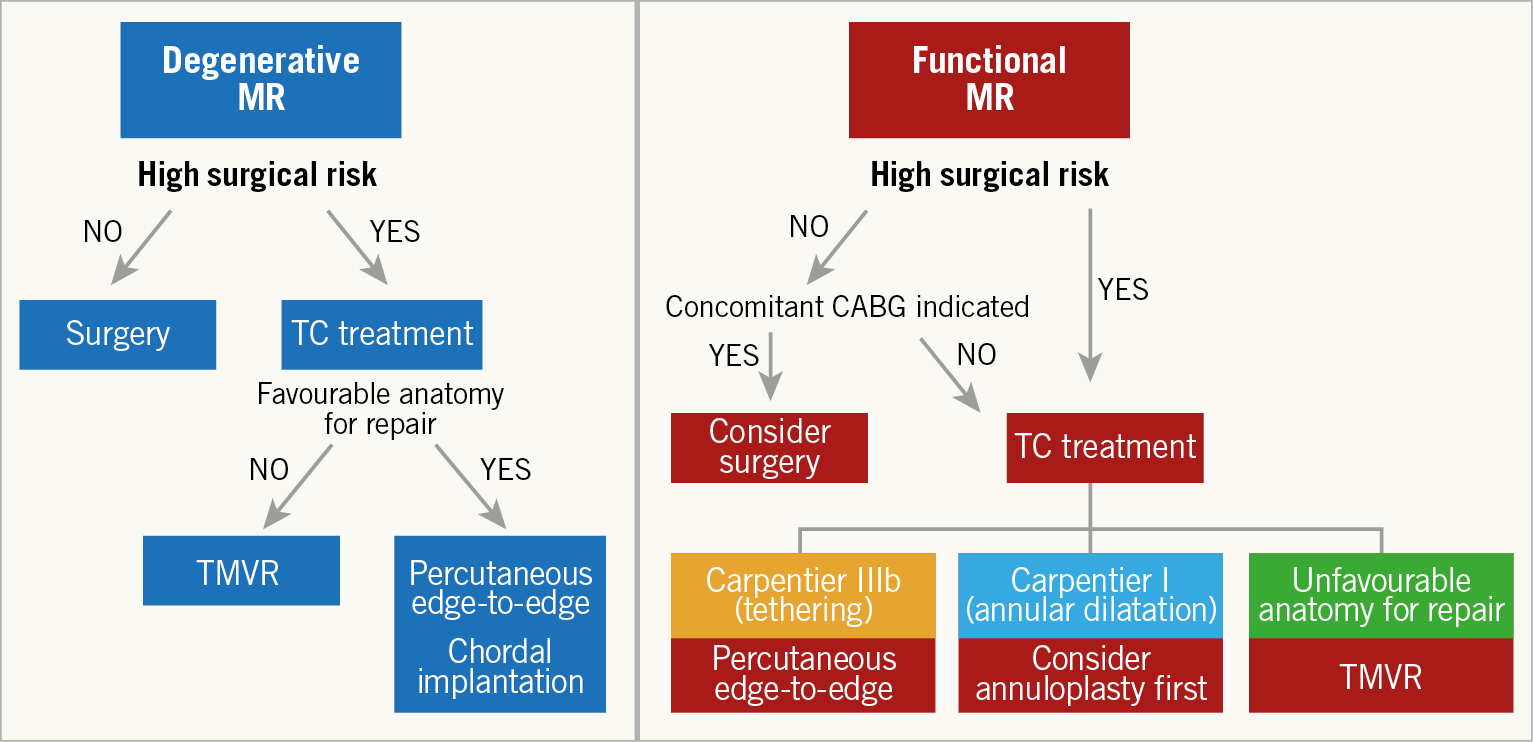
Figure 1. Algorithm to select the most appropriate therapeutic strategy in both DMR and FMR patients according to the clinical profile. CABG: coronary artery bypass grafting; DMR: degenerative mitral regurgitation; FMR: functional mitral regurgitation; TC: transcatheter; TMVR: transcatheter mitral valve replacement
DEGENERATIVE MITRAL REGURGITATION
The gold standard treatment for severe DMR is surgical valve repair, which is usually performed through minimally invasive access3.
When surgical risk is high, transcatheter MV therapies should be considered. Available options are edge-to-edge repair or artificial chordal implantation. In case of unsuitable anatomy for repair, TMVR is an alternative.
In the setting of transcatheter therapies, the anatomical suitability for MV repair derives from the surgical experience and from the results of specific trials4,5,6,7.
FUNCTIONAL MITRAL REGURGITATION
In this clinical context, the valve leaflets and chordae are not primarily affected; usually they are structurally normal or mildly abnormal and the MR results from an imbalance between closing and tethering forces on the valve, secondary to LV alterations. In the Carpentier surgical classification, FMR can be type I (main mechanism is annular dilatation) or type IIIb (left ventricular [LV] disease).
The outcomes of surgery in isolated FMR are largely suboptimal, due to the relatively high risk of morbidity and mortality8,9,10. Moreover, no evident benefits of repair over replacement have been shown, due to the high incidence of recurrent MR in case of repair11. Therefore, transcatheter therapies play a predominant role in FMR treatment.
Different transcatheter repair options are available for FMR, including edge-to-edge repair, annuloplasty devices and valve replacement.
Several trials have been designed to understand the role of transcatheter repair in FMR12,13,14,15,16,17,18.
The recent contrasting results from the MITRA-FR and COAPT trials clearly showed that FMR is a very heterogeneous disease and several factors should be considered for predicting the outcomes of an intervention in this setting17,18.
The crucial importance of patient selection (anatomical and clinical) may be suggested by the positive results shown in the COAPT trial. According to the neutral MITRA-FR study, it may be speculated that the prognostic benefit of MitraClip® (Abbott Vascular, Santa Clara, CA, USA) implantation is absent in inappropriately selected patients17.
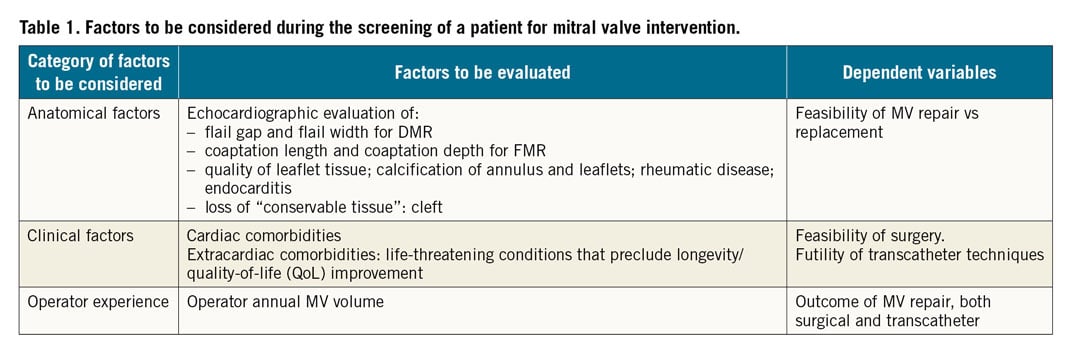
Table 1 summarises the most important categories of factors to be considered in the multidisciplinary evaluation of MV interventions for DMR and FMR.
REPAIR-FIRST STRATEGY – “A GOOD REPAIR IS BETTER THAN A REPLACEMENT…”
During the screening of a possible candidate for mitral intervention, the feasibility of repair is usually the first element to be considered. Transthoracic and transoesophageal echocardiography are the gold standard diagnostic methods to assess the feasibility of a “good repair”. There are a number of reasons supporting why a repair strategy should come first, rather than a replacement one, mainly related to the following safety issues.
– The 30-day mortality rate after TMVR seems higher than those reported in major studies for other mitral transcatheter repair therapies, suggesting a higher safety profile for repair as compared to replacement. The overall 30-day mortality rate reported in a recent pooled analysis conducted in 272 patients treated with seven different replacement devices was 13%19.
– Repair is more respectful of the physiology of the mitral valve complex, since the impact of the implant is minimal. The implantation of a prosthetic valve is associated with a non-physiological inflow pattern, which results in increased left ventricle (LV) stress that can be detrimental in the long term, especially in patients with a reduced ejection fraction. Moreover, the fixation of a prosthesis to the mitral annulus might negatively affect the contraction of the basal part of the LV20,21,22.
– The life expectancy of a patient implanted with a prosthetic valve is reduced, due to valve-related events (thromboembolic and haemorrhagic events, and the risk of prosthetic valve endocarditis)23,24,25. Valve thrombosis represented a major issue in the initial phase of the development of TMVR, especially for valves implanted in a supra-annular position, due to alteration in the atrial flow dynamics. The duration of anticoagulation after TMVR has not yet been determined, but it is likely that patients receiving TMVR will require long-term anticoagulation (perhaps even life-long). The risk of structural valve deterioration is another element to take into consideration.
– In relation to procedural access, while most repair procedures are performed through a transseptal approach, the large majority of TMVR have so far been performed through transapical access. Although the feasibility of transfemoral transseptal implantation has been reported and will be the preferred method in the future, transapical access remains (at the moment) the most used approach. Although in the largest series of transapical TMVR reported so far the complication rates related to the access were negligible (only 1%, with no intraprocedural death)26, this represents a major disadvantage of TMVR compared to repair.
– Last but not least, at this stage there are obvious logistical reasons favouring a repair-first approach. Since no TMVR device is commercially available so far, the screening process to get a patient approved and included in a feasibility trial is generally relatively long. This may create discomfort or even be dangerous in case of severely symptomatic patients, especially if an alternative is immediately available. Moreover, at the moment, the rate of screening failure with the different TMVR systems is high due to anatomical reasons (mainly annular size and risk of LVOT obstruction) and to have a patient denied treatment after a long waiting period can be frustrating for patients and physicians alike. The consequence is that TMVR is considered as a second step only if a good repair is deemed to be unfeasible.
“…BUT A REPLACEMENT IS BETTER THAN A BAD REPAIR”: THE IMPORTANCE OF THE PROCEDURAL PERFORMANCE
The main advantage of TMVR is that it is usually associated with a constant and reproducible reduction of MR. While percutaneous repair therapies have demonstrated effectiveness and safety in the majority of patients, in the real world approximately one fourth to one third of patients who undergo treatment with a variety of these therapies will continue to have at least moderate residual MR, which has been associated with increased rates of mortality and rehospitalisation27,28. Apart from anatomical feasibility, the technical performance of the operating team (interventionists and imagers) is also crucial, especially in more complex anatomies. The concept of “mitral centres of excellence” is well known in surgery, where it has been observed that outcomes and the chance of a durable repair are related to the institutional volume29,30. Recently, the same concept has been shown in the interventional arena, where procedural outcomes after a MitraClip procedure are also strictly related to the institutional volume, in terms of safety and efficacy, suggesting that mitral valve intervention should be performed in centres of excellence with high expertise, similar to what has been done in surgery for several years31. In particular, increasing institutional experience was associated with improvements in procedural success, procedure time, and procedural complications and the impact of institutional experience was greater when considering the goal of achieving optimal MR reduction. Improvements were evident after about 50 cases, with continued improvements observed up to 200 cases.
Figure 2 summarises the specific factors affecting the outcome of a MitraClip procedure to be considered in the patient’s candidacy for the procedure, including clinical and anatomical factors, as well as the expertise of the operating team.
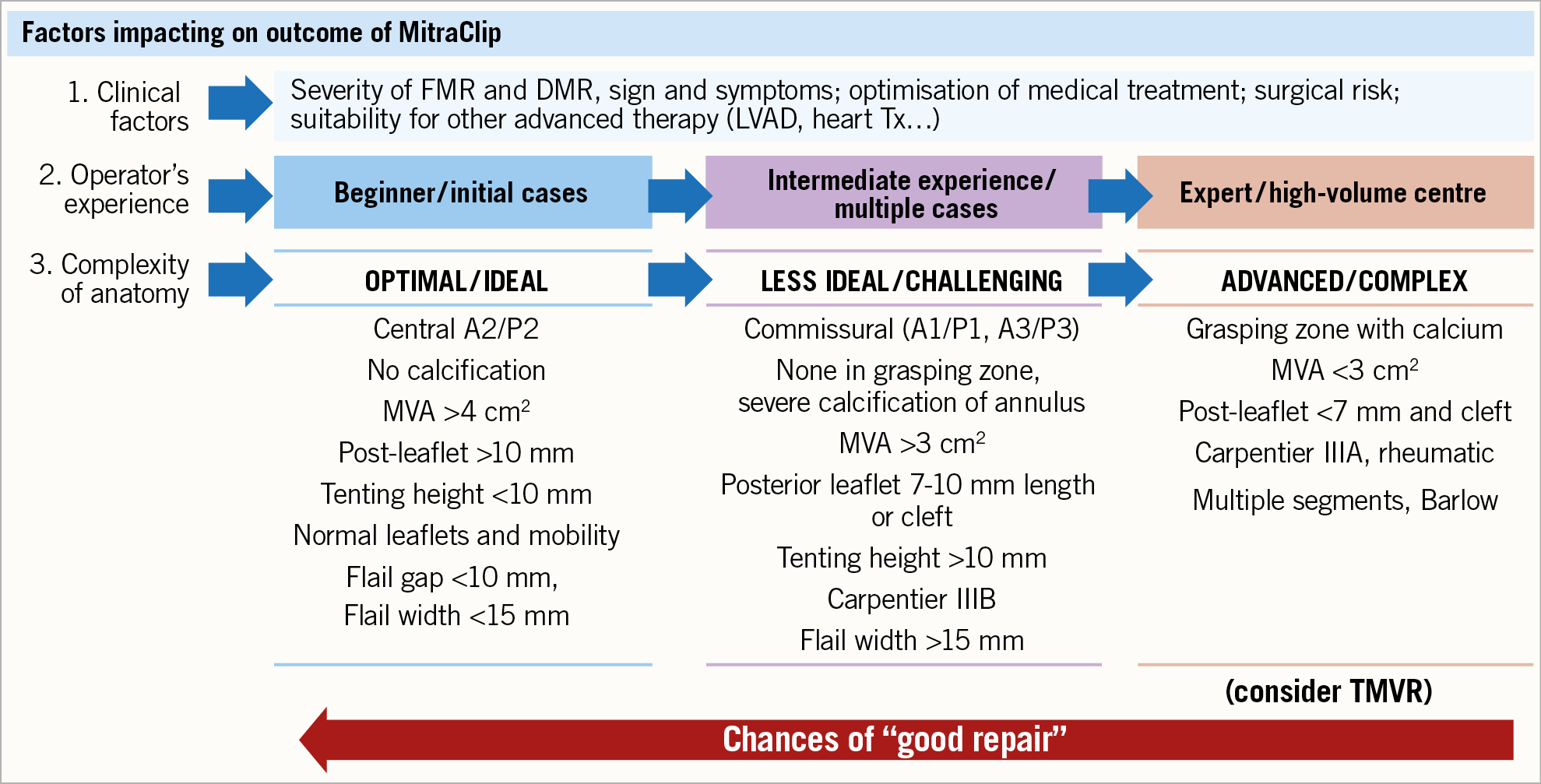
Figure 2. Factors affecting outcome of MitraClip implantation to be considered in the patients’ candidacy to procedure. Clinical factors are only the first step; operator experience should always be considered since this allows fixing the complexity of different anatomies. The patients presenting with the characteristics listed in the third column should be considered for replacement.
If the chances of a good repair are reduced due to unsuitable anatomy or insufficient institutional experience, valve replacement has to be considered as an alternative.
One argument in favour of the “repair-first” strategy is that some of the repair techniques keep the option for subsequent valve replacement open32. The aspect of a future intervention has to be considered in the decision-making process when choosing the initial repair strategy, since some repair techniques (especially annuloplasty) have minimal impact on eventual subsequent valve replacement. Therefore, in cases where TMVR is anatomically feasible, leaving a “bad” clip behind should be avoided, although the feasibility of TMVR has been reported even after edge-to-edge repair33.
In general, keeping in mind that residual MR has an impact on outcomes even in the short term, if a “bad repair” is expected, the best option is to consider the patient for replacement, avoiding the risk of two procedures rather than a single one.
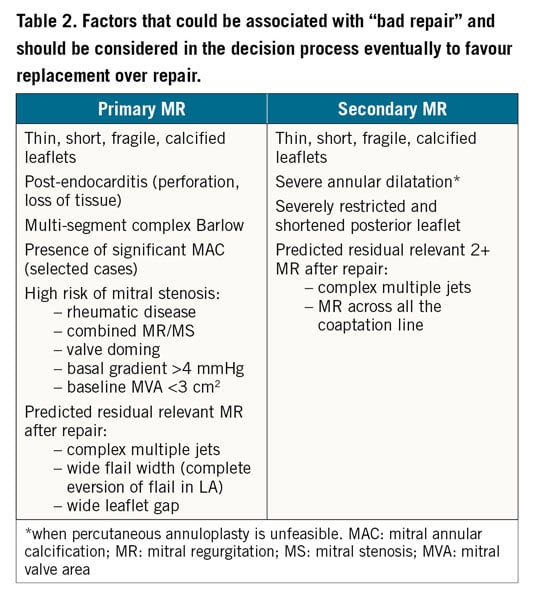
Table 2 shows the factors that should be taken into consideration to favour replacement over repair.
SPECIFIC CRITERIA FOR SUCCESS OF TRANSCATHETER MV REPAIR ACCORDING TO CURRENT EVIDENCE
Data from trials and post hoc observational studies suggest that patient selection for a mitral valve repair system should include the following.
1. Anatomy of the MV: to predict procedural success of both repair and replacement and durability of the results in case of repair; in particular, patients with unfavourable anatomy for good and durable valve repair should eventually be considered for replacement. Patients in whom replacement is deemed to be anatomically not feasible will ultimately be eligible only for repair or for conservative treatment or for surgery, if operable34,35.
Anatomical selection of patients should take into account the following.
– Accurate evaluation of the location and extension of the lesion using 2D and 3D TEE; A2/P2 lesions represent the anatomy associated with the highest chance of successful repair with both leaflet and chordal approaches; for DMR a flail width ≤15 mm (primary MR) and a flail gap <10 mm (primary MR) are supposed to constitute the ideal anatomy for successful MitraClip procedure in DMR; annular dimension has to be carefully considered in case of chordae implantation, since the mismatch between the leaflet lengths and the anteroseptal annular diameter has been associated with increased risk of MR recurrence36.
For patients with FMR, the presence of sufficient leaflet tissue for mechanical coaptation constitutes the most important factor; a coaptation length more than 2 mm and coaptation depth less than 11 mm are supposed to be ideal for MitraClip success in FMR. In the presence of severe annular dilatation, an annuloplasty repair could be considered as the first choice.
– Leaflet length and integrity: the presence of perforation or heavy leaflet calcification is usually a contraindication for repair. Presence of a deep cleft-like indentation should also be evaluated, since it may affect the outcome of repairs.
– Baseline MV area; final transvalvular gradient affects long-term outcomes as well as the residual MR, but baseline MVA is no longer a “strong” criterion for patient selection. The TVT registry data did not show a significant association of preprocedural mitral valve area <4 cm², mitral annular calcification, or mitral valve gradient >4 mmHg with procedural success28.
– Annular size, calcifications and proximity of the circumflex artery: more specifically for annuloplasty devices, the assessment of the annular size and the presence of annular calcifications are crucial for assessing the feasibility of transcatheter annuloplasty. It is also important to assess the proximity of the circumflex artery to the mitral annulus, which may preclude the feasibility of the procedure. An angio-CT scan is a fundamental tool to assess all of this information.
2. Clinical factors: to define a candidate who can still profit from the correction of the valve problem and who is under proper guideline-directed medical therapy (GDMT); to define the procedural risk based on associated co-pathologies and associated cardiac conditions (this last evaluation is also essential in DMR).
3. Timing-related factors: to identify the correct window of opportunity for the treatment, excluding end-stage patients who are eventually candidates for advanced heart failure (HF) therapy (such as cardiocirculatory support or heart transplantation). Importantly, if possible, patients should not be treated in the acute phase of HF (which has been associated with the worst outcome), but should first be stabilised37.
Differently from anatomical factors, clinical and timing-related factors are obviously more important to define the benefit of any therapy rather than decide the type of procedure.
EVALUATION OF SUITABILITY FOR TMVR: THE “CHIMERA” OF ONE DEVICE FITS ALL
If, after the screening echocardiography, the patient is deemed not to be a candidate for a “good repair”, further evaluation with an angio-CT scan is mandatory to assess the feasibility of TMVR. A CT scan provides a variety of crucial information for patient eligibility and procedural planning, including sizing of the mitral annulus, assessment of the predicted neo-LVOT with an estimation of the risk of LVOT obstruction, evaluation of the coronary status, presence and distribution of annular calcifications, planning of the procedural access and evaluation of the fluoroscopic working plan during the intervention.
One of the most important limitations of TMVR is patient eligibility. The concept that one replacement device could fit all the anatomo-functional mitral variations is appealing but, for the moment, it remains theoretical. It is important to understand that at this stage only a limited number of patients are anatomically eligible for TMVR, with a screening failure rate reported in the different series of up to 65%26,38, mainly due to annular size and too small neo-LVOT. If the risk of LVOT obstruction based on preprocedural CT is high, prophylactic septal alcohol ablation or laceration of the anterior mitral leaflet by means of LAMPOON techniques should be considered in selected cases39,40.
Different sizes of prosthesis and improved designs are under investigation in order to increase the number of eligible patients.
Once anatomical suitability has been confirmed, two important clinical elements have to be considered in the decision-making process for TMVR: the general condition and the frailty status of the patient should be good enough to tolerate a transapical procedure (which, at the moment, is the most used approach) and the patient should be able to tolerate long-term oral anticoagulation.
Figure 3 shows a proposal for a simplified flow chart to guide the decision-making process to identify patients for repair and replacement.
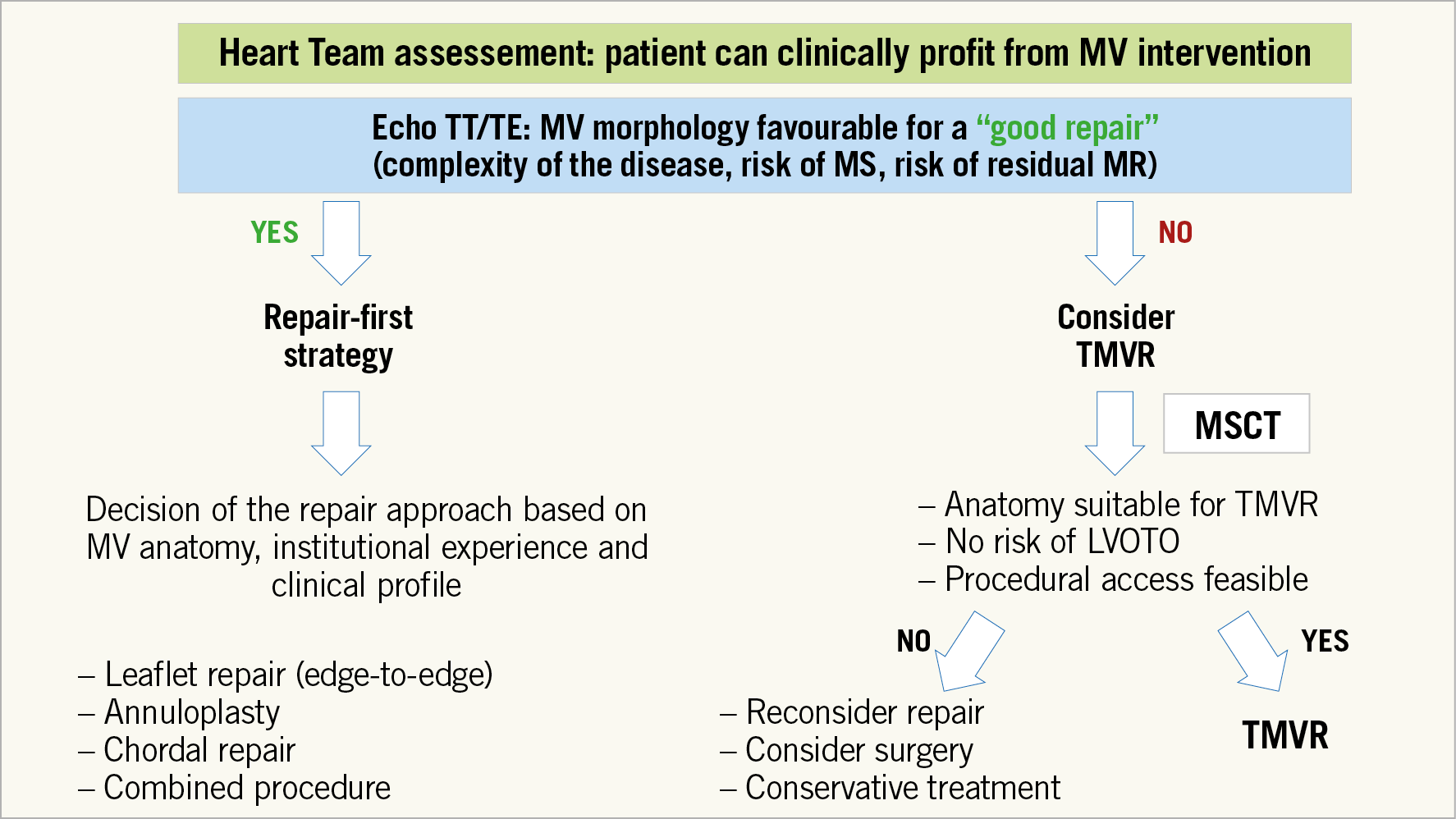
Figure 3. Proposal for a simplified flow chart to guide the decision-making process to identify patients for repair and replacement in those who are candidates for transcatheter mitral procedures. MR: mitral regurgitation; MS: mitral stenosis; MSCT: multislice computed tomography; MV: mitral valve; TE: transoesophageal; TMVR: transcatheter mitral valve replacement; TT: transthoracic
Conclusion
As has previously occurred in the surgical field, selecting transcatheter mitral repair or replacement is becoming a concrete therapeutic choice for the treatment of high-risk patients with severe mitral regurgitation. The two options will most likely be complementary rather than competitive, considering the complexity and variability of mitral valve disease. Although at the current stage a repair-first strategy seems to be the most appropriate approach, there are a number of patients who are suboptimal candidates for a “good repair” who will probably profit better from TMVR. Finding the sweet spot in mitral valve treatment requires a tailor-made approach for each patient with an accurate evaluation of valve disease anatomy and clinical presentation. In addition, the treatment should be performed in a dedicated valve centre with a high level of expertise in mitral valve interventions.
Conflict of interest statement
M. Gavazzoni is a consultant for Biotronik. G. Nickenig reports honoraria for lectures or advisory boards from Abbott, AGA, AstraZeneca, Bayer, Berlin Chemie, BioSensus, Biotronik, BMS, Boehringer Ingelheim, Daiichi Sankyo, Edwards, Medtronic, Mitratech, Novartis, Pfizer, Sanofi Aventis, St. Jude; participation in clinical trials for Abbott, AGA, AstraZeneca, Bayer, Berlin Chemie, BioSensus, Biotronik, BMS, Boehringer Ingelheim, Daiichi Sankyo, Edwards, Medtronic, Mitratech, Novartis, Pfizer, Sanofi Aventis, St. Jude; research funding from DFG, BMBF, EU, Abbott, AGA, AstraZeneca, Bayer, Berlin Chemie, BioSensus, Biotronik, BMS, Boehringer Ingelheim, Daiichi Sankyo, Edwards, Medtronic, Mitratech, Novartis, Pfizer, Sanofi Aventis, and St. Jude. F. Maisano is a consultant for Abbott Vascular, Medtronic, Edwards Lifesciences, Perifect, Xeltis, Transseptal Solutions, Magenta and Cardiovalve, has received grant support from Abbott Vascular, Medtronic, Edwards Lifesciences, Biotronik, and Boston Scientific, has received royalties from Edwards Lifesciences and 4Tech, and is co-founder of Transseptal Solutions, 4Tech, and SwissVortex. M. Taramasso reports consultancy fees from Abbott Vascular, Edwards Lifesciences, 4Tech, Boston Scientific, CoreMedic, Occlufit, and SwissVortex, outside the submitted work.

