
Mitral regurgitation (MR) has numerous aetiologies, but may broadly be classified as either primary MR (PMR; also termed degenerative or organic MR) or secondary MR (SMR; also termed functional MR)1. PMR arises from structural changes in the valve itself or supporting structures, whereas the principal derangement in SMR is global or regional left ventricular dysfunction due to ischaemic or non-ischaemic cardiomyopathy resulting in apical and lateral distraction of the papillary muscles and subsequent tethering of the mitral leaflets with lack of coaptation. In SMR, the mitral valve complex is structurally normal until late in its course when annular dilatation and leaflet fibrosis may occur. Less commonly, SMR arises from annular dilatation due to left atrial enlargement (usually a consequence of atrial fibrillation). The prognosis of patients with PMR and SMR depends on the severity of the MR, the degree of left ventricular (LV) remodelling and dysfunction, the presence of pulmonary hypertension and right ventricular dysfunction, and associated clinical comorbidities.
Although there have been no randomised trials, for many years surgical mitral valve repair has been the standard of care for patients with severe PMR1,2,3 due to studies demonstrating high success rates, infrequent recurrence, symptomatic improvement and the re-establishment of an expected age and sex-matched trajectory of survival4. In contrast, mitral valve repair or replacement surgery has not been shown to improve the prognosis of patients with severe SMR5; isolated mitral surgery is rarely performed for SMR6. Rather, guideline-directed medical therapy for heart failure (HF) and cardiac resynchronisation therapy (CRT) are the foundation therapies for most patients with SMR1,2,3.
Over the last decade, several randomised trials have been performed that have now established transcatheter mitral valve repair with the MitraClip® (Abbott Vascular, Santa Clara, CA, USA) as the preferred treatment for selected patients with both PMR and SMR. Based on a predicated surgical technique7, the MitraClip procedure is performed from a transvenous approach with fluoroscopic and transoesophageal guidance and re-approximates the anterior and posterior mitral leaflets in systole with one or more clips, creating a double orifice or bow-tie configuration to reduce MR. The MitraClip was first rigorously evaluated in the EVEREST II randomised trial, being compared to surgical mitral valve repair in low operative risk patients8. Among 273 randomised patients, 73% had PMR and 27% had SMR. As expected, the MitraClip was safer than surgery, but surgery was more effective at reducing MR. At the time this trial was performed, operators were not yet experienced with the MitraClip and procedural success rates were low (77%), necessitating mitral valve surgery in the majority of failed cases for effective treatment. However, a significant interaction was noted between the primary effectiveness outcome and the type of MR such that surgery was superior to MitraClip in PMR but not SMR8. Thus, following EVEREST II, surgical mitral valve repair remained the standard of care for operative candidates with severe PMR. However, on the basis of registries in high surgical risk patients in which the MitraClip resulted in symptomatic improvement9, the MitraClip was approved for use in the USA for patients with PMR at prohibitive risk for surgery.
The role of the MitraClip in patients with HF and severe SMR has recently been clarified by two randomised trials comparing this device with medical therapy. Among 302 patients enrolled in the MITRA-FR trial, the MitraClip did not improve the rates of death, hospitalisation for HF or the composite of death or HF hospitalisation at one year10. In contrast, among 614 HF patients with severe SMR enrolled in the COAPT trial, treatment with the MitraClip resulted in marked two-year reductions in death and HF hospitalisations, as well as improved quality of life and functional capacity11. In this trial, the numbers of patients needed to treat to prevent one HF hospitalisation and one death were three and six, respectively, representing a profound absolute risk reduction.
Three theories have been proposed to explain the different results between MITRA-FR and COAPT. Perhaps the most cogent relates to differences in the severity of SMR and LV dysfunction of the patients enrolled in these trials. MITRA-FR defined SMR according to European echocardiographic consensus criteria12, which allowed lesser degrees of volumetric regurgitant flow than the US echocardiographic criteria13 which were used to qualify patients for COAPT. As such, the severity of SMR as reflected in the mean effective regurgitant orifice area was less in MITRA-FR than in COAPT (mean 0.31±0.10 cm2 vs 0.41±0.15 cm2, respectively)10,11. In addition, COAPT capped the size of the LV end-systolic dimension for enrolment at 7 cm, whereas MITRA-FR had no such restriction. As such, the mean LV end-diastolic volume was greater in MITRA-FR compared with COAPT (135±35 mL/m2 vs 101±34 mL/m2, respectively)10,11. Thus, the haemodynamic impact of the degree of MR relative to the LV chamber size was substantially greater in patients enrolled in COAPT than in MITRA-FR. Grayburn and colleagues have described this difference conceptually by noting that COAPT enrolled patients predominantly with disproportionately severe SMR, whereas most MITRA-FR patients had proportionately severe or non-severe SMR14.
Second, patients in MITRA-FR were treated with background therapies of commonly used HF medications, the doses of which were allowed to vary in both groups10. In contrast, patients in COAPT were enrolled only after treatment with all maximally tolerated doses of such medications as confirmed by an independent central eligibility committee, and there were few major medication changes during follow-up11. The impact of these differences is reflected in the symptomatic status of the control groups which improved more during the course of the study in MITRA-FR than in COAPT10,11. Finally, the number of clips per patient was greater in COAPT than MITRA-FR (despite the smaller LV size), contributing to a higher rate of acute procedural success and sustained reduction in MR over time10,11.
Thus, COAPT and MITRA-FR were complementary studies informing treatment decisions as to which patients with HF and SMR are likely to benefit (or not benefit) from MitraClip treatment. To date, no patient subgroup in COAPT has been identified that did not derive clinical benefit. After a detailed review of the data, in March 2019 the US FDA approved the MitraClip for treatment of SMR in HF, with labelled indications carefully following the COAPT inclusion criteria.
On the basis of these studies in PMR and SMR, we can thus provide evidence-based recommendations as to the utility and appropriate uses of transcatheter leaflet approximation with the MitraClip – who, what, when, where, and why (Figure 1). In PMR, the MitraClip is indicated to provide symptomatic benefit for patients too high risk to undergo effective surgical mitral valve repair. Ongoing studies will determine if the risk-benefit ratio of the MitraClip warrants expanding its use to those at intermediate surgical risk. In SMR, the MitraClip is indicated to improve prognosis and quality of life in patients meeting COAPT criteria, namely where: 1) the patient is symptomatic (New York Heart Association Class II, III or ambulatory IV) despite use of all maximally tolerated guideline-directed medical therapies for HF, and CRT and revascularisation if appropriate; 2) severe echocardiographic SMR due to ischaemic or non-ischaemic LV cardiomyopathy is present as assessed using either the COAPT integrative multiparametric approach12 or American Society of Echocardiography criteria13; 3) LV ejection fraction is 20%-50% and LV end-systolic volume is ≤7 cm; 4) severe pulmonary hypertension and right ventricular dysfunction are absent. Future studies will determine if additional patients may benefit from MitraClip correction of SMR, such as those more or less clinically ill or with moderate MR before LV remodelling has ensued.
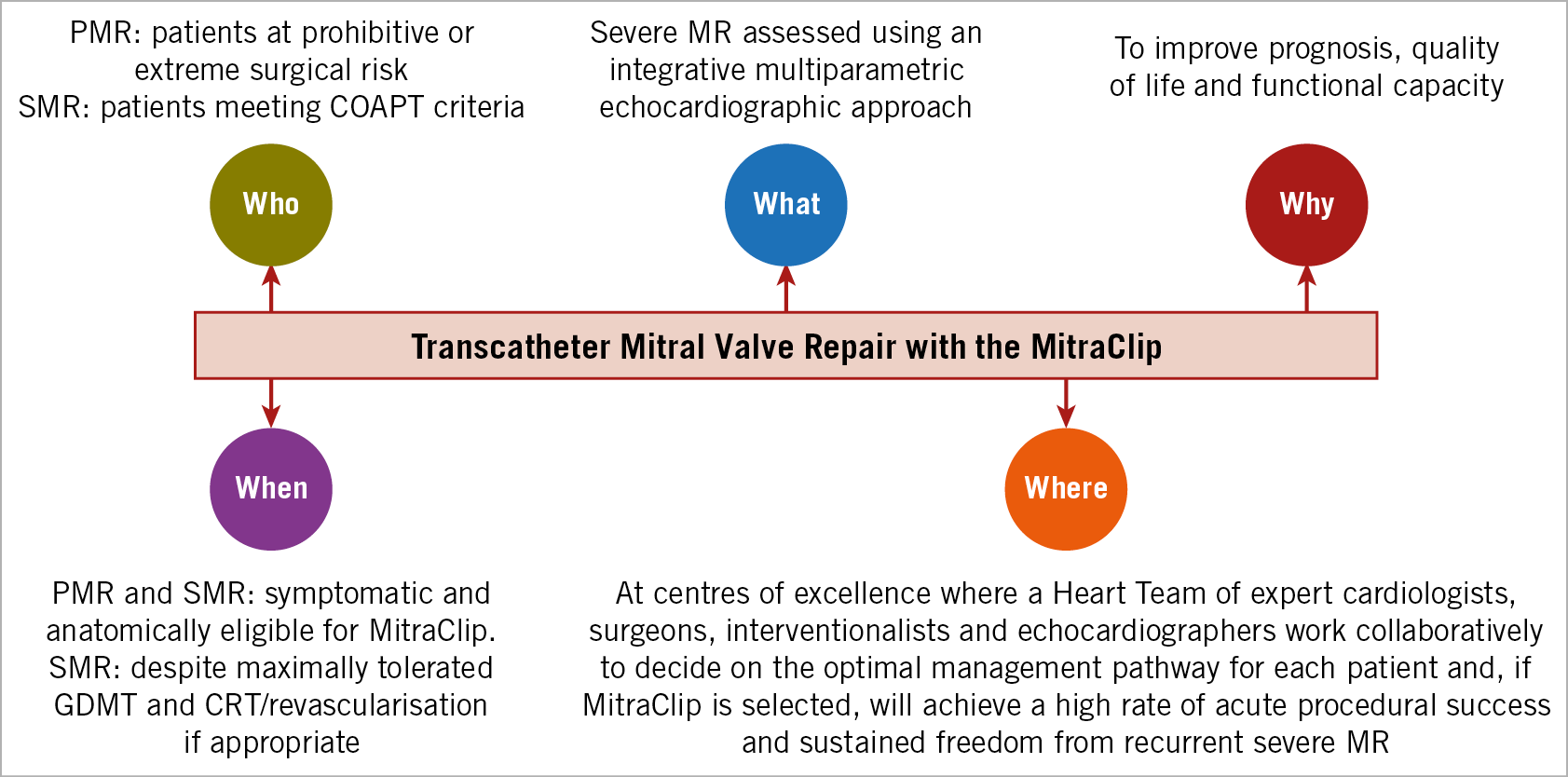
Figure 1. The Who, What, When, Where, and Why of transcatheter mitral valve repair with the MitraClip. In addition to the guidelines in this Figure, each patient’s clinical comorbidities and specific anatomic features should be taken into account by the Heart Team for informed shared decision making with the patient. CRT: cardiac resynchronisation therapy; GDMT: guideline-directed medical therapy; MR: mitral regurgitation; PMR: primary mitral regurgitation; SMR: secondary mitral regurgitation
In addition, MitraClip treatment of both PMR and SMR should be performed at centres with the institutional, echocardiographic and interventional operator expertise in order to ensure success rates of >90%, the requirements for which are only now emerging15. Finally, treatment decisions must be conditioned on the specific clinical conditions and ventricular and valvular anatomy of each patient. Whether the optimal treatment is mitral valve surgery, MitraClip therapy, medical therapy or enrolment into an investigational protocol of a novel mitral valve device must be considered by a multispecialty Heart Team comprised of (at a minimum) cardiologists specialising in valvular disorders and HF, mitral valve surgeons and interventionalists, and echocardiographers, considering each patient’s preferences and goals for therapy16.
Conflict of interest statement
Within the last 3 years G. Stone has received speaker honoraria from Terumo and Amaranth; served as a consultant to Shockwave, Valfix, TherOx, Reva, Vascular Dynamics, Robocath, HeartFlow, Gore, Ablative Solutions, Matrizyme, Miracor, Neovasc, V-wave, Abiomed, Claret, Sirtex, Ancora, SpectraWave, Qool Therapeutics, MAIA Pharmaceuticals and Orchestra Biomed, and has owned equity or options in Ancora, Qool Therapeutics, Cagent, Applied Therapeutics, Biostar family of funds, MedFocus family of funds, SpectraWave, Orchestra Biomed, and Aria. O. Alfieri has no conflicts of interest to declare.

