
The first two randomised controlled trials on secondary MR (MITRA-FR and COAPT) have recently been published1,2. These two studies had an excellent quality of follow-up modality with few lost patients, confirming the poor prognosis of this disease. The safety of MitraClip implantation (equivalent in both studies if we use the same definition of complications) and its efficacy to decrease mitral regurgitation (MR) (around 95% procedural success defined as residual MR ≤2+) are confirmed but we have to understand why two studies with apparently similar designs led to such different clinical results.
Several reasons could explain why MITRA-FR is negative:
– It is an academic study in a large population (ejection fraction [EF] from 15% to 40%) with severe MR according to the European Guidelines (effective regurgitant orifice area [EROA] ≥20 mm2)3.
– The design of the protocol, which was published three years before the results, had never been changed.
– The medical treatment was optimised for the duration of the study per “real-world practice”, leading to real maximum potential benefit in both the control and the treated groups.
– In the absence of a central eligibility committee, some patients were possibly included at a very advanced stage, when it was too late.
Several reasons could explain why COAPT is positive:
– It was designed to test the MitraClip in its ideal target, a very selected population with less depressed ventricular function and more severe MR according to the ACC/AHA Guidelines4.
– The final design of the protocol (published two months before the presentation of the results) had been adapted throughout the inclusion period (inclusions of patients increased from 350 in 2012 to 610 in October 2016).
– A central eligibility committee checked any inclusion before randomisation, leading to a homogeneous selected population.
– This regulatory driven study was supported by the industry.
– The medical treatment at baseline was significantly better in the MitraClip group than in the guideline-directed medical therapy (GDMT) group, and the physicians were discouraged from modifying the treatment during the follow-up.
In addition to offering the first large and high-quality assessment of MitraClip, COAPT and MITRA-FR highlight the difficulty of drawing clear conclusions and creating guidelines due to the vast heterogeneity of patients within the secondary mitral regurgitation (SMR) population. In their retrospective analysis of a large cohort, Kortlandt et al5 conclude that there is a clinical benefit to the use of MitraClip treatment in an SMR population. This study has the benefit of comparing the three available therapeutic options in real-world practice, including surgery (the surgical option has often been forgotten in recent publications). However, the same issue of population heterogeneity is a concern in this study and the challenge continues to understand which subset of the population will benefit most from percutaneous treatment or surgery.
An analysis integrating ventricular parameter values into the interpretation of the MR severity has been proposed6. Actually, despite similar left ventricular ejection fraction (LVEF) (33.3±6.5 vs. 31.3 in MITRA-FR and COAPT, respectively), the severity of regurgitation was higher in the COAPT study (0.31 vs. 0.41 cm2) due to the reference to different guidelines - European versus ACC/AHA. However, for both guidelines, a severe SMR remains defined as a regurgitation fraction (RF) ≥50%. Thus, the threshold value of regurgitant volume corresponding to an RF ≥50% could be calculated, integrating personal ventricular parameters with a simple formula: RVolcalc = 50% (LVEF x EDV). Using this formula, RVol >39 ml in the MITRA-FR study and >30 ml in the COAPT study would have been considered as severe. In MITRA-FR the mean initial RVolmeasured was 45 ml. The RVolmeasured is not given in the COAPT study; however, hypothesising a similar VTIMR in both studies, the RVolmeasured would be 60 ml in this study (RVolcalc = VTIMR × EROAcalc).
Thus, in the COAPT study, the RVolmeasured was 196% the threshold value defining a severe MR, whereas in MITRA-FR it was only 115%. A three-dimensional representation of this calculation is provided in Figure 1.
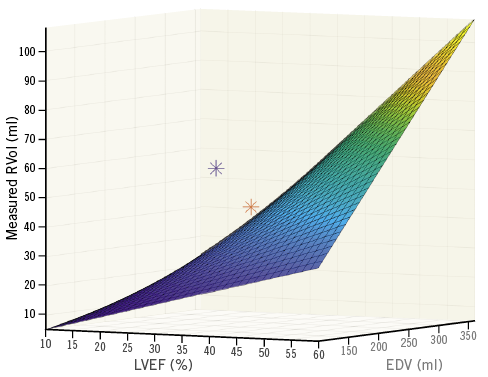
Figure 1. Three-dimensional graphical representation of the MITRA-FR (red star) and COAPT study (blue star) integrating the values of their regurgitant volume (RVol, in ml), left ventricular ejection fraction (LVEF, in %) and end-diastolic volume (EDV, in ml). The curve represents the value of RVol corresponding to an RF ≥50, integrating the EDV, and the LVEF.
Therefore, the benefit of treating an SMR depends mainly on the initial MR severity with regard to the ventricular parameter.
The different selection process in the two studies explains the apparently different results.
– MITRA-FR, that represented more of a real-life population with its wide inclusion criteria for MitraClip therapy, led to disappointing results.
– COAPT focused on very selected patients with smaller ventricles and more severe MR (as screened by an experienced Heart Team) in whom the correction of MR saves lives and limits the rehospitalisation rate.
Moreover, the definition of severe MR should be revisited regarding the ventricular parameter.
Acknowledgements
The authors thank the Fulbright Program, Fédération Française de Cardiologie, the Philippe Foundation, and the Institut Servier Doctoral Fellowship program for their support. The authors thank Alexis Bruhat who created Figure 1.
Conflict of interest statement
J.F. Obadia was the Principal Investigator of the MITRA-FR study, is a consultant for Abbott, Medtronic, Landanger, Delacroix-Chevalier, has received research support from Boehringer Ingelheim, Abbott, Medtronic, and Edwards, consulting fees/honoraria from Edwards, Abbott, Medtronic, Servier, and Novartis, and royalty income from Landanger, and Delacroix-Chevalier. E. Donal has received research support from General Electric, and Abbott, and consulting fees from Bristol-Myers Squibb, and Novartis. D. Grinberg has no conflicts of interest to declare.

