Abstract
Secondary mitral regurgitation (MR) has a complex pathophysiology that includes global or segmental left ventricular (LV) motion abnormalities (of non-ischaemic or ischaemic origin) leading to impaired leaflet coaptation of a normally structured mitral valve (MV). In this context, the LV functional and geometrical changes result in MV leaflet tethering, MV annulus flattening and the decrement of systolic MV closing forces. In light of its complexity, management of secondary MR remains a challenge. In fact, a long-lasting successful treatment using a single medical device and/or intervention that addresses solely the MV target cannot, at least at the present time, be proposed.
Procedural details and clinical data
The benefits of MV surgery in the treatment of secondary MR remain controversial and, as a result, only a third of patients with secondary severe MR are referred to surgery, and secondary severe MR is present in 90% of the patients who are denied surgery1. Since the introduction and popularisation of percutaneous MV repair by means of the MitraClip® system (Abbott Vascular Inc., Menlo Park, CA, USA), many patients with secondary MR have been treated. In reality, although satisfactory acute results are reported, short-term follow-up re-hospitalisation for heart failure remains at over 20%2,3.
We herein summarise a possible algorithm that focuses not only on the MV, but also on the failing LV. Our aim is to define the possible aetiologies of secondary MR, interrupting the vicious circle of LV volume overload, LV dilatation and secondary MR.
– Myocardial ischaemia is the most frequent aetiology of secondary MR. The possibility of a percutaneous coronary intervention that may improve myocardial perfusion, and consequently global and/or segmental LV function, should be investigated first and before addressing percutaneously the MV anatomy.
– Once the coronary status has been clarified, whenever secondary MR is accompanied by depressed LV function (LVEF ≤35%), the presence of left bundle branch block (LBBB) and QRS duration should be investigated. Cardiac resynchronisation therapy (CRT) should be considered, whenever indicated, before approaching percutaneously the failing MV. In fact, severe secondary MR improves in more than 50% of patients after CRT, and the benefits have been demonstrated even in patients with LVEF ≤40% and QRS duration ≥120 ms4,5. CRT reduces the amount of secondary MR through LV reverse remodelling, myocardial contractility improvement, papillary muscle resynchronisation, MV leaflet tethering force reduction and closing force increases, and MV annular contraction improvement6.
– Moreover, the cardiac rhythm should also be considered whenever staging patients for percutaneous treatment of secondary MR. In fact, the presence of atrial fibrillation (AF) and ventricular dyssynchrony secondary to LBBB have been demonstrated to worsen the degree of LV dysfunction and the prognosis of patients with ischaemic dilative cardiomyopathy and secondary MR7,8. In a surgical cohort of patients treated for secondary MR, De Bonis et al have demonstrated that concomitant successful surgical ablation of AF and/or CRT provided an additional symptomatic and prognostic advantage, when compared to MV restrictive annuloplasty alone9. It is reasonable to think that, when sinus rhythm is regained together with ventricular synchrony, the benefits in terms of LV function improvement and LV dimension reduction are more pronounced. In this context, the afterload mismatch, resulting acutely after the MR correction, can be overcome more easily by a better performing LV. In light of these findings, a staged catheter-based ablation of AF should be considered when dealing with secondary MR.
– When looking more specifically at the percutaneous techniques to repair secondary MR, we should review critically the importance of addressing the MV annulus. In surgical experience, it is clear that omission of ring annuloplasty, when performing the “edge-to-edge” repair, is associated with suboptimal results10. Most probably, the excessive tension exerted on the approximating MV stitch may affect repair durability. There is experimental evidence using force transducers in the animal model suggesting that MV annular geometry predominates in determining the tension in the “edge-to-edge” repair11. In fact, peak tension occurs in diastole and is directly proportional, mainly to the MV septolateral annular diameter11. As a consequence, in patients with secondary MR, it is unreasonable to think that consistent long-term results will be reached by the sole application of the MitraClip. In fact, it is advisable that a durable percutaneous MV repair should include, in the near future, placement of a prosthetic MV ring to allow for homogeneous distribution of stress resulting from annular reshaping and reduction of septolateral annular diameter.
– Until percutaneous annuloplasty becomes a standard of practice, we have developed some technical tips to treat secondary MR by means of MitraClip. We believe that in these patients MV clipping should be started at the commissural level (posterior-medial) where the gap between the leaflets is reduced and fibrous tissue enhances grasping and lowers the risk of tearing. The aim of percutaneous MV repair is to recreate a coaptation line by multiple adjacent, parallel and equally distanced clips (sometimes up to five clips are necessary) which should be placed in a “medial to lateral” sequence (Figure 1). Such an “aggressive” clipping should always be performed while monitoring with echocardiography the degree of regurgitant volume, the residual MV effective orifice area and the transvalvular gradient.
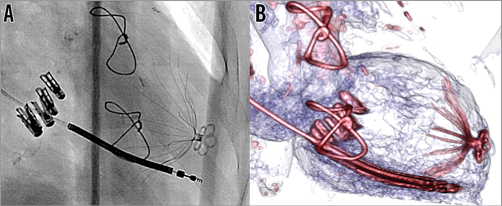
Figure 1. Combined percutaneous treatment of secondary mitral regurgitation. A) Intraoperative fluoroscopy with 4 MitraClips in place recreating a new coaptation line, LV partitioning device at the LV apex, and AICD lead in a patient suffering with secondary MR, heart failure and LV apical aneurysm. B) Pre-discharge cardiac CT with perfect anchoring of the LV partitioning device (Parachute®; CardioKinetix, Menlo Park, CA, USA) and the newly placed MitraClips recreating a new coaptation line.
– When treating secondary MR, we should consider that the timing of the intervention plays a key role in guaranteeing sustained success. In fact, in patients who are treated at a too far advanced stage, even optimal MV repair may fail, as a result of further LV and annular dilatation. Although the issue of MV repair versus replacement in patients with secondary MR has remained controversial for many years, in a recently reported trial from the Cardiothoracic Surgical Trials Network (CTSN) it clearly emerges that recurrence of at least moderate MR at one year is substantially greater with MV repair compared with replacement (32.6% vs. 2.3%, p<0.001)12. In general, valve-sparing MV replacement is nowadays preferred in patients with the most severe degrees of MV leaflet tethering, as determined by a tenting height >11 mm, a posterior leaflet angle >45 degrees, and/or a basal LV aneurysm or dyskinesis12-15. In the near future, the availability of percutaneous MV prostheses will represent a preferable alternative for those selected candidates with advanced changes in MV and LV geometry and function.
– Finally, a global percutaneous approach to secondary MR should keep in consideration the possibility of mechanically reducing the LV volume to lower myocardial wall stress and, in the long term, to enhance a further reduction in LV volume. Although this hypothesis is not fully supported by the surgical literature, a totally percutaneous approach to LV reshaping could achieve more encouraging results without the heavy perioperative morbidity and mortality burden. For this reason, in patients with secondary MR, if a well-defined area of ventricular dyskinesia is identified in the middle-apical portion of the ventricle, we always consider placement of an LV partitioning device (Figure 1)16. It should be emphasised that the existing LV partitioning devices are able to exclude only a limited part of the weakened ventricle and, for this reason, cannot fully prevent further ventricular enlargement. The newest technology in the field of percutaneous ventricular reshaping devices should allow for an extended LV reshaping.
Conclusion
Percutaneous treatment of secondary MR is an evolving field that requires timely knowledge of the existing treatment technology and strategic timing for its application. Although the results of percutaneous MV repair in this complex group of patients are encouraging, longer follow-up is required. There are three ongoing prospective randomised trials (COAPT, RESHAPE-HF and MITRA-FR) investigating the MitraClip in secondary MR. These trials will clarify whether a therapy purely aimed at reducing secondary MR may improve prognosis in patients with LV dysfunction. As already emphasised, we suspect that the progression of LV dilatation, although slower, will continue, leading to further MV annular dilatation. To anticipate this condition, a more structured and holistic approach should be proposed to tackle, in a percutaneous and safer fashion, the various cardiac conditions that lead to the complexity of secondary MR.
Conflict of interest statement
The authors have no conflicts of interest to declare.

