
CASE SUMMARY
BACKGROUND: A 74-year-old man with end-stage heart failure and biventricular systolic dysfunction was admitted for cardiogenic shock. The functional mitral regurgitation was severe with difficult valve characteristics and the cardiogenic shock was refractory to the initial medical treatment.
INVESTIGATION: Physical examination, transthoracic and transoesophageal echocardiography.
DIAGNOSIS: Refractory cardiogenic shock complicating end-stage heart failure in a patient with biventricular systolic dysfunction and severe mitral regurgitation with difficult valve characteristics.
MANAGEMENT: Intra-aortic balloon pump (IABP), MitraClip.
KEYWORDS: biventricular failure, cardiogenic shock, functional mitral regurgitation, intra-aortic balloon pump (IABP), MitraClip
PRESENTATION OF THE CASE
A 74-year-old male was admitted to our hospital because of heart failure (HF) with anasarca. He had a history of ischaemic cardiomyopathy secondary to a previous myocardial infarction (1992), with a “frequent flyer” status during the last year.
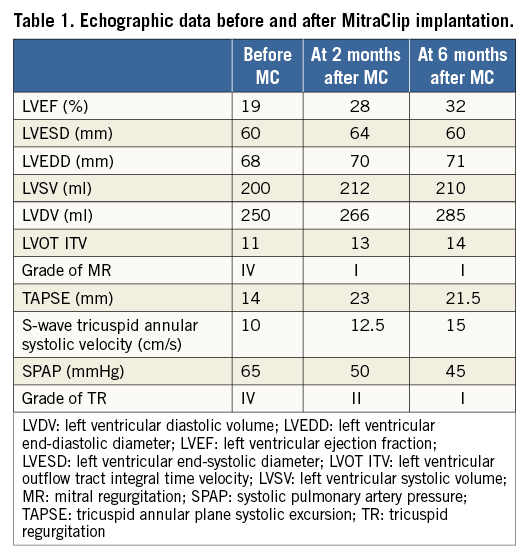
A transthoracic echocardiogram (TTE) revealed grade IV mitral regurgitation (MR), a severe left (LVEF=19%) and right ventricular dysfunction (Moving image 1), with high pulmonary blood pressure and grade IV tricuspid regurgitation (Figure 1, Table 1).
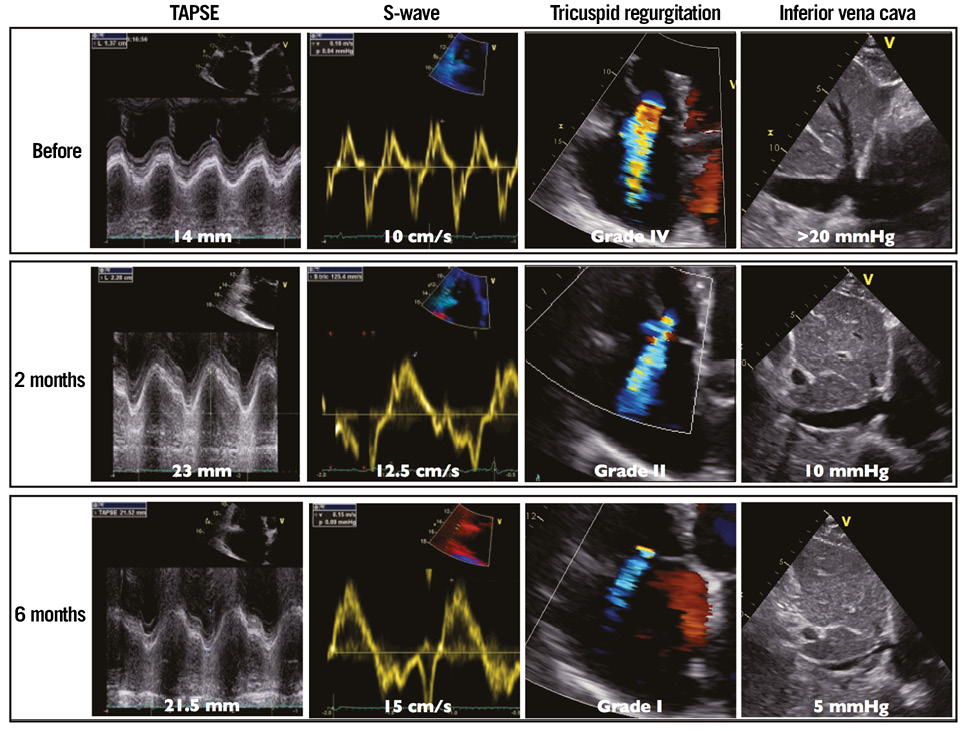
Figure 1. Right ventricular systolic function, tricuspid regurgitation before MitraClip implantation and during follow-up.
Symptoms were considered to be related to end-stage HF with poor prognosis. His age, comorbidities (renal failure, chronic obstructive pulmonary disease [COPD] on long-term oxygen therapy) and advanced biventricular failure contraindicated left ventricular assist device (LVAD) or surgical treatment of MR. Transoesophageal echocardiography (TEE) showed an asymmetric tethering with localisation at the lateral part of the leaflets (Figure 2, Moving image 2, Moving image 3). This tethering mainly concerned the posterior leaflet with a high degree of tethering angle and a large defect of coaptation.
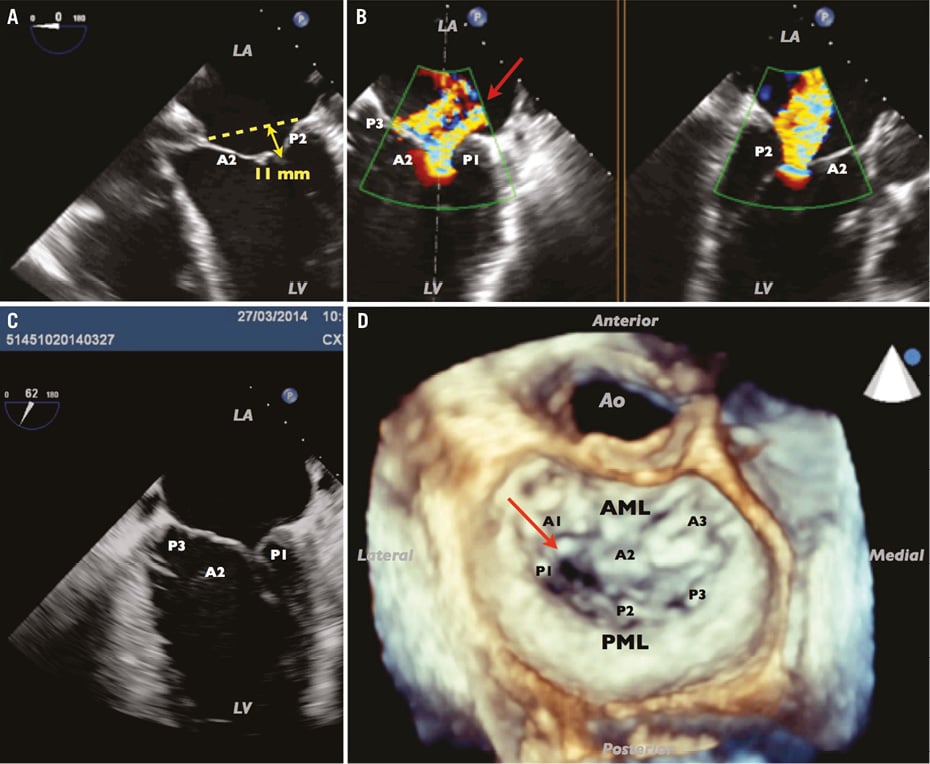
Figure 2. Mechanisms of MR. Global tenting at 11 mm (A), two jets of regurgitation (B) with a main lateral jet (red arrow) corresponding to the main tethering on the lateral parts of both leaflets (C), with a large defect of coaptation (red arrow) on 3D (D). AML: anterior mitral leaflet, segmented into A1, A2 and A3; LA: left atrium; LV: left ventricle; PML: posterior mitral leaflet, segmented in P1, P2 and P3
After improvement on the optimal medical therapy, and because of difficult mitral valve characteristics, a too advanced phase of HF and the high procedural risk, the Heart Team decided to delay the decision on a possible MitraClip insertion.
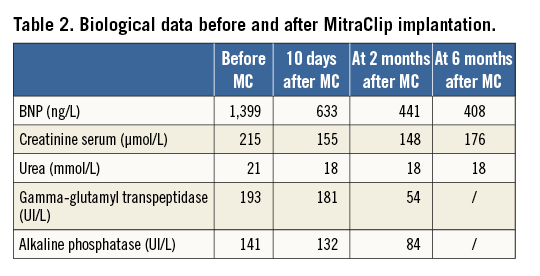
Two months after the index admission, he remained hospitalised for the same symptoms. Baseline brain natriuretic peptide (BNP) and creatinine serum values were, respectively, 1,399 ng/L and 215 µmol/L (Table 2). Despite intravenous diuretic treatments (furosemide 1 gr/24 hrs), haemodynamic instability with oliguria required an inotropic support (dobutamine). Despite increasing doses (15 µg/kg/min), the patient was considered to be “sliding fast” because of persistent haemodynamic instability.
How should I treat this refractory cardiogenic shock in a patient with chronic biventricular heart failure and mitral regurgitation with difficult valve characteristics?
How would I treat?
THE INVITED EXPERTS’ OPINION

This case illustrates the challenge of MitraClip therapy in a patient with severe secondary mitral regurgitation (MR) with very advanced left ventricular (LV) dysfunction and shock.
Such a complex situation must be discussed by a Heart Team including clinicians, echocardiographists, interventionists, heart failure specialists, and surgeons1,2 .
Mitral valve surgery is not an option here3. The patient’s age and numerous comorbidities argue against cardiac assist whilst awaiting heart transplantation or chronic LV assist device implantation4.
Thus, the choice would be either MitraClip® (Abbott Vascular, Santa Clara, CA, USA) implantation or palliative therapy.
The ESC/EACTS recommendations on the management of valvular heart disease state that MitraClip therapy may be considered in symptomatic patients with severe primary or secondary MR despite optimal medical therapy, who fulfil the echo criteria of eligibility, are judged by a Heart Team to be inoperable or at high risk for surgery, and who have a life expectancy greater than one year (recommendation Class IIb, level of evidence C)1. On the other hand, the ACC/AHA 2014 guidelines do not recommend the use of MitraClip in patients with secondary MR following the decision of the FDA2.
When considering MitraClip implantation in this patient, the question is whether the intervention will be more “utile than futile”, taking into account his life expectancy and the possibility of improvement.
The presence of multiple comorbidities such as severe chronic obstructive pulmonary disease requiring oxygen therapy at home for a long time is a serious adverse factor for TAVI and the same holds true for MitraClip implantation4,5.
As regards “cardiac outcomes”, there is still an ongoing debate on the effectiveness of any intervention on the mitral valve in patients with severe MR and severe LV dysfunction. Experimental data suggest that mitral valve repair has no effect on LV positive remodelling in such cases6. The same has also been demonstrated after surgical valve repair3,7.
MitraClip therapy is mostly used in secondary MR in the real world, but data on patients with severe LV dysfunction are limited8-14. They suggest that the procedure may be safe; however, cases of low cardiac output have been reported15. No data show a benefit in terms of survival but the current experience suggests a temporary improvement in symptoms which may be related to an increase in cardiac output. The patient here has many predictors of poor outcome: age >75 years, NYHA Class IV, renal failure, extreme LV dysfunction and a dilated LV, severe tricuspid regurgitation and right ventricular failure12,14,15. In addition, the two months which have elapsed since the first discussion on MitraClip implantation have made any improvement even more unlikely.
The anatomy would also be challenging for MitraClip implantation due to the apparently severe leaflet tethering and the asymmetric location of the MR jet. However, this anatomy is “potentially suitable” in experienced hands16. Aortic balloon contrapulsation could be used during the procedure, even if there is no proof of its efficacy in this setting.
The final decision in this patient will thus require a comprehensive Heart Team discussion including the patient and family. The MitraClip could be presented at most as an option with the aim of transiently improving symptoms.
Conflict of interest statement
A. Vahanian is a consultant for Abbott Vascular, Edwards Lifesciences and Valtech. D. Himbert is a consultant and proctor for Edwards Lifesciences and Medtronic Inc. E. Brochet has received speaker’s fees from Abbott Laboratories. P. Nataf has no conflicts or interest to declare.
How would I treat?
THE INVITED EXPERTS’ OPINION

Prompt diagnosis and subsequent treatment of cardiogenic shock in patients with chronic heart failure of ischaemic origin involves a number of onerous challenges. In particular, in patients with secondary mitral valve regurgitation (MR) the difficulties go well beyond the technical gesture of “fixing” the failing valve.
We will summarise our thoughts in the following paragraphs.
1. In this situation, before considering any sort of mitral valve intervention, we would try to restabilise perfusion pressure by means of inotropic support (preferably dobutamine) and vasoconstrictors (norepinephrine) and gently unload the failing heart by means of renal replacement therapy.
2. In cardiogenic shock patients who are refractory to catecholamines, we would consider perfusion of calcium sensitisers such as levosimendan. In fact, this drug improves myocardial performance by increasing myofilament calcium sensitivity and does not impact on intracellular calcium and AMP concentrations, with very little impact on myocardial oxygen consumption17.
3. Although an intra-aortic balloon pump (IABP) may seem the most agile and least invasive form of support, its real benefits are questionable. In the context of ischaemic (acute myocardial infarction) cardiogenic shock, the IABP-SHOCK II trial has clearly shown no significant acute or long-term benefits in terms of reduced morbidity and mortality in patients managed with IABP18. For this reason, other forms of biventricular support should be considered.
4. Extracorporeal membrane oxygenation (ECMO) offers the advantage of unloading both ventricles, providing complete cardiopulmonary support. During the intraprocedural phases, the left ventricular unloading can reduce the extreme mitral valve tethering, enhancing mitral valve leaflet grasping, and facilitating percutaneous mitral valve repair. The results of mitral valve “clipping” can be tested in real time, intraoperatively, by slowly reducing the ECMO support and allowing controlled left ventricular loading19.
5. In the case reported herein, once the initial cardiogenic shock picture had been improved and stabilised pharmacologically, the authors procrastinated, for two additional months, regarding the treatment of severe secondary MR. Although we understand perfectly their doubts and hesitance in addressing ab initio this challenging MV anatomy, we would have initiated a prompter intervention as soon as the haemodynamic profile had stabilised.
6. Since the introduction of the MitraClip, a broader application of this technology has been proposed in complex anatomical scenarios which goes well beyond the initially presented EVEREST criteria20.
7. In our experience with percutaneous treatment of severe MR in patients with advanced ischaemic dilatative cardiomyopathy, the instrumentation required for a MitraClip procedure under sedation does not necessarily exacerbate and precipitate the already fragile haemodynamic equilibrium of the left and right ventricles. Having said that, although we would not have started a priori a form of mechanical circulatory support, we would certainly have considered the possibility of having a “stand-by” ECMO support available before starting the MitraClip procedure. Normally, guidewires are left within the controlateral groin vessels and, in this way, expeditious percutaneous cannulation and ECMO initiation can be achieved only in case of catastrophic haemodynamic derangement.
8. In many such patients MitraClip implantation remains, at least at present, the last resort which could offer satisfactory acute outcomes at a negligible perioperative risk. We have adopted a specific strategy to approach secondary mitral regurgitation with extreme leaflet tethering and coaptation deficit. To start with, we would perform a rather anterior-inferior (in relation to the fossa ovalis) interatrial septal puncture. A low puncture can facilitate reaching the tethered coaptation line that finds itself well below the mitral annular plane. When a wide systolic coaptation gap is present, adequate simultaneous grasping of both leaflets may be difficult. In this anatomical condition, we consider adopting what we have previously described as the “zipping technique”21. The concept behind this technique is to achieve a new coaptation line progressively using a series of parallel clips starting from the posteromedial commissure and moving laterally. Clipping should be started in the “less tethered area”, possibly closer to the fibrous tissue of the commissure (in case the tethering is symmetrically extended towards the commissures), to guarantee an initial good anchoring and approximation of the leaflets. The following clips should be adjacent and evenly spaced, including a similar amount of tissue on both the anterior and posterior leaflet, to distribute the stretching forces homogeneously and prevent tissue tearing. Placement of the clips should mimic a suture line and should be aimed at annihilating the residual mitral regurgitation. During the alignment of the MitraClip devices, 3D transoesophageal echocardiography should be used to monitor the resulting mitral valve effective orifice area and transvalvular gradient.
9. Once the percutaneous mitral valve repair has been completed, we would evaluate timely the blood shunt generated through the newly created atrial septal defect. In fact, in patients with biventricular failure, an excessive left to right shunt can challenge the residual function of the right ventricle. In this case, we would not hesitate primarily to close the septostomy with a percutaneous occluder device.
10. Our considerations have to be interpreted within the status quo. Treatment of advanced secondary mitral valve regurgitation by means of MitraClip therapy may carry a heavy burden of recurrent MR and readmission for heart failure, especially when the mitral valve anatomical derangements are associated with left ventricular dilatation and dysfunction, such as in the case presented here20. In the near future, more advanced forms of percutaneous mitral valve repair (addressing the annulus as well) and/or percutaneous mitral valve replacement may become routinely available and, at that stage, would represent a reasonable option to address such complicated clinical and anatomical scenarios.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
This challenging case illustrates the issue of MitraClip insertion in a critically ill patient and the need for a strategy adapted to the MR and valve characteristics.
As the haemodynamic condition was unstable, a MitraClip insertion with intra-aortic balloon pump (IABP) support was conducted as a rescue therapy.
The procedure was performed under general anaesthesia. The first clip was placed on the central portion of the leaflets to improve coaptation with residual grade III (Figure 3, Moving image 4). A second clip was placed beside the first with a final grade I (Figure 3, Moving image 5).
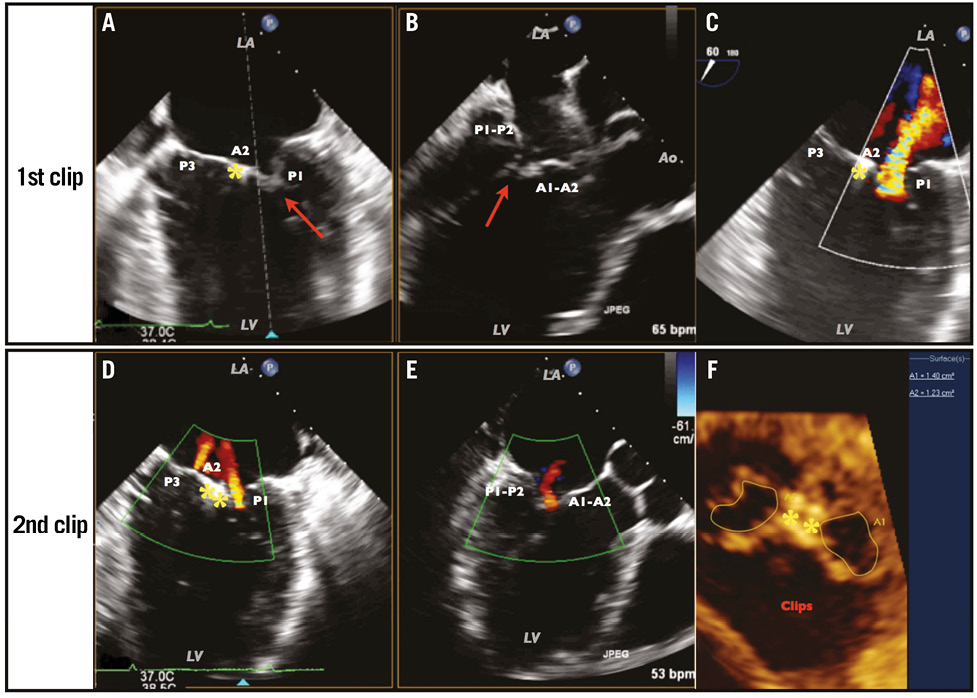
Figure 3. Result after implantation of the first clip in the mid portion of the leaflets, and the final result after implantation of the second clip. After the first clip: persistent tethering of the lateral parts of the leaflets is visualised in the mid-commissure view (A) and LVOT view (B), leading to a significant defect (red arrows) in coaptation with grade III MR (C). Final result achieved after the second clip: a multiplanar view showing the mid-commissure (D) and LVOT (E) views, confirming a grade I MR with two minor jets in each orifice. Multiplanar reconstruction of the double orifice area (F), measuring a total area of 2.62 cm2. Yellow stars indicate the clips.
Weaning from mechanic ventilation was achieved within six hours after completion of the procedure and from the IABP within 30 hours, by reducing the trigger ratio. Dobutamine and intravenous diuretics could be withdrawn, respectively, at three and five days after the procedure. The patient was discharged 10 days afterwards, with a significant reduction of HF signs and B-type natriuretic peptide (BNP) levels as well as improved renal function (Table 2).
During the six months of follow-up, the patient was well with NYHA Class I+. Long-term oxygen therapy could be reduced from 20 to six hours per day. BNP and creatinine were further decreased, and cholestatic hepatitis was normalised. TTE showed a stable MR grade, an improved LVEF (Moving image 6) and stroke volume with persistent end-diastolic dilatation of the left ventricle. Remarkably, right ventricular systolic function had improved with decreased pressures and a regression from grade IV to I of tricuspid regurgitation (Figure 1, Table 1).
MitraClip therapy has previously been described as an alternative treatment for MR22,23. Franzen et al have shown clinical benefit at six months for patients with end-stage systolic HF after procedural success24. However, mortality and MR recurrence in patients with a too advanced phase of HF are still high15. The indication for MitraClip therapy is still debated and conservative medical therapy may be preferred for critically ill patients15,24. Otherwise, a few cases have been reported in emergency25,26, but not in the case of biventricular failure and inevitable clinical course towards “crash and burn”.
Indeed, MitraClip therapy can be critical, especially because of the risk of an afterload mismatch27. It is responsible for a sharp decrease in the postoperative LVEF27. Despite the use of inotropic support, MACE rates are high when LVEF is <25%, and the decrease of LVEF after MitraClip implantation is still a predictor of mortality27,28. Moreover, in cardiomyopathy with a lack of contractile reserve and very low LVEF, the IABP is probably the best option to reduce afterload. The controlled weaning-off by reduction of the pump’s trigger enabled the postoperative period to be tolerated well.
These surprising improvements highlight the real impact of the MR reduction in midterm follow-up, even in a patient with an advanced phase of chronic biventricular cardiomyopathy. However, the insufficient reverse of the maladaptive process with an ongoing LV dilatation (Table 1) emphasises the risk of MR recurrence in the longer term15. Despite the fact that MitraClip therapy is better for the preservation of the right ventricular function than surgery29, a worsening can occur when significant pulmonary hypertension is present30. However, this marked improvement showed that management may improve the global HF.
This unexpectedly significant improvement was possible because of the optimal result of the procedure. Indeed, asymmetric tethering, a high degree of posterior leaflet tethering and a large defect of coaptation can make grasping difficult, and MitraClip implantation failure might have occurred with a strategy of the target zone on the origin of the jet. Therefore, a strategy with a first clip to stabilise the leaflets and to improve the defect of coaptation31, before completely correcting the coaptation with a second clip, should be preferred.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. 2D TTE: large four-cavity view, before MitraClip implantation.
Moving image 2. 3D TEE: surgical view of the mitral valve and the functional MR.
Moving image 3. 3D TEE with colour Doppler: surgical view of the mitral valve with functional MR.
Moving image 4. 2D TEE multiplanar view: result after implantation of the first clip with grade III persistent MR.
Moving image 5. 3D TEE: surgical view of the final result.
Moving image 6. 2D TTE: large four-cavity view at 6 months after implantation of the MitraClip.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. 2D TTE: large four-cavity view, before MitraClip implantation.
Moving image 2. 3D TEE: surgical view of the mitral valve and the functional MR.
Moving image 3. 3D TEE with colour Doppler: surgical view of the mitral valve with functional MR.
Moving image 4. 2D TEE multiplanar view: result after implantation of the first clip with grade III persistent MR.
Moving image 5. 3D TEE: surgical view of the final result.
Moving image 6. 2D TTE: large four-cavity view at 6 months after implantation of the MitraClip.

