
Abstract
Aims: Transcatheter mitral valve implantation (TMVI) is a novel approach that may enable a less invasive effective reduction of mitral regurgitation (MR). A limitation of the MitraClip is that definitive implantation of a clip precludes future therapy with TMVI. The purpose of this paper is to describe contemporary treatment considerations in patients with mitral valve regurgitation.
Methods and results: In this report we describe an attempted MitraClip implantation which resulted in no reduction of MR severity. There was a consensus that additional clips would probably not be effective. MitraClip implantation was therefore abandoned and the clip was removed, allowing subsequent successful TMVI with the Tiara™ system three weeks later. Echocardiography revealed secure seating of the prosthesis with good mitral valve function, trivial paravalvular leakage and transvalvular gradient of 3 mmHg. The patient recovered rapidly and was discharged four days post implant.
Conclusions: The clinical approach towards high-risk patients with significant MR may change in the next few years. In selected patients, in whom an initial attempt with MitraClip implantation results in only limited efficacy, the clip may be retrieved during the index procedure to allow subsequent TMVI.
Introduction
Mitral regurgitation (MR) is the most common valvular disease1. The prevalence of severe MR increases in elderly patients and increased longevity is projected to expand the disease burden further2. The optimal treatment for patients with severe symptomatic MR is either surgical valve replacement/repair or medical treatment3,4. However, many patients with MR are at high risk for conventional surgery, while medical treatment is commonly ineffective5-7. Recently, less invasive strategies for MR reduction have been developed8-14. MitraClip (Abbott Vascular, Santa Clara, CA, USA) implantation is currently the most commonly utilised transcatheter therapy for severe MR. Clinical trials show that, although MitraClip implantation is relatively safe, an effective and durable outcome is frequently not achieved15-18. Transcatheter mitral valve implantation (TMVI) is a novel approach that may, theoretically, enable a more effective reduction in MR severity19-22. A significant concern is that a transcatheter valve cannot be implanted after a MitraClip has been deployed and released.
Case description
A 39-year-old male with dilated cardiomyopathy, resulting in severe left ventricular dysfunction, and severe functional MR had decompensated heart failure. Echocardiographic evaluation revealed left ventricular (LV) ejection fraction of 16%, LV end-diastolic diameter of 94 mm, LV end-diastolic volume of 328 cc and severe functional MR. A transoesophageal echocardiogram (TEE) confirmed severe functional MR with poor leaflet coaptation and a vena contracta of 8 mm with an effective regurgitant orifice area of 0.44 cm2. The patient was declined for conventional surgery because of his comorbidities. The suitability of the mitral annular anatomy for TMVI was assessed by pre-procedural CT22,23. Mitral annular dimensions as well as landing zone characteristics were assessed to ensure annular dimensions within the range of the anticipated valve size as well as non-interference with anchoring mechanisms (Figure 1). The risk of LVOT obstruction was excluded using device simulation with the CT data set, as recently described23 (Figure 1). The patient had a projected mitral valve area of 1,003 mm² with a septal to lateral distance of 31 mm and an intercommissural distance of 38 mm. There was no annular calcium and a sufficient posterior shelf was noted. All these parameters seemed compatible with TMVI; however, the patient was referred for MitraClip implantation as a first-line treatment of high-risk MR patients who seem to be good candidates for the MitraClip.
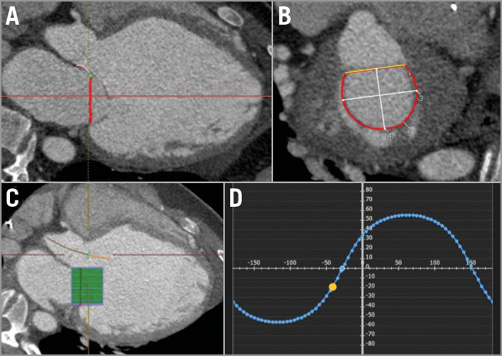
Figure 1. Computed tomography pre-procedural assessment for transcatheter mitral valve implantation. A) & B) There was no annular calcium and sufficient posterior shelf noted. The D-shaped mitral annulus was measured with a projected valve area of 1,003 mm², a septal to lateral distance of 31 mm and an intercommissural distance of 38 mm. C) The risk of LVOT obstruction was excluded using device simulation. D) Curve of perpendicularity to the mitral valve.
MitraClip implantation was attempted in August 2014. Clip capturing of both anterior and posterior leaflets did not reduce MR (Figure 2, Moving image 1). There was a consensus that additional clips would probably not be effective as the gap between the leaflets in this extremely dilated left ventricle was very wide. A decision was made to abort MitraClip implantation, ungrasp the leaflets, remove the device and pursue a transcatheter valve option as the patient was already considered to be a good candidate for TMVI.
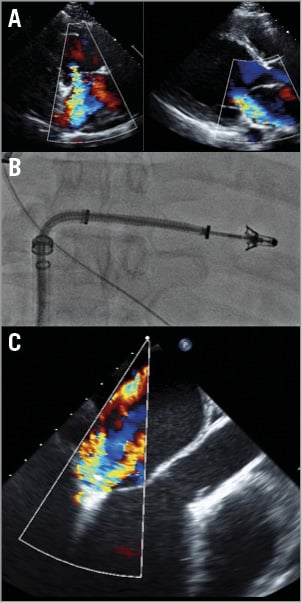
Figure 2. MitraClip implantation. A) Echocardiographic images show a severely dilated left ventricle with severe mitral valve regurgitation. B) MitraClip deployment. C) Repeated attempts to clip the A2 and P2 segments did not result in a significant reduction in mitral regurgitation. It was apparent that additional clips would not be effective and the clip was removed.
Three weeks after the MitraClip attempt, in September 2014, TMVI was performed under general anaesthesia with endotracheal intubation in a hybrid operating room. The TMVI procedure was approved on compassionate grounds by Health Canada and informed consent was obtained. The Tiara™ system (Neovasc Inc., Richmond, BC, Canada) is a catheter-based self-expanding trileaflet bovine pericardial tissue mitral bioprosthesis, designed to fit an asymmetric D-shaped mitral annulus24-27. A hydrophilic wire was inserted through the right jugular vein and into the coronary sinus to guide as an anatomical landmark for device positioning with fluoroscopy (Figure 3A). A mini anterior thoracotomy was performed to access the left ventricular apex. The delivery system was inserted into the left ventricle and across the mitral valve into the mid left atrium (Moving image 2). This action was followed by release of the atrial portion (Figure 3B, Figure 3C, Moving image 3). Orientation of the flat portion of the D-shaped valve to the left ventricle outflow tract, coaxiality, and alignment were confirmed with three-dimensional TEE. After full deployment of the atrial skirt, the valve was rotated until the flat portion of the D-shaped frame corresponded with the outflow tract. Seating of the valve onto the atrial annulus was achieved by gentle traction towards the left ventricle. Rapid ventricular pacing at 180 beats/min was initiated, and the ventricular anchoring mechanism was released with capture of the anterior and posterior native mitral leaflets (Figure 3D-Figure 3F, Moving image 4). A post-implant ventricular angiogram showed no significant MR, and invasive pressure measurement confirmed no gradient across the outflow tract. TEE revealed secure seating of the prosthesis with normal valvular function, trivial paravalvular leakage, and a transvalvular gradient of 3 mmHg (Figure 4, Moving image 5, Moving image 6). The patient was haemodynamically stable throughout the procedure. There were no apical access issues, and blood transfusion was not required. He was extubated in the operating room and transferred in a stable condition to the surgical intensive care unit. An increase in systemic pressure and a reduction of pulmonary pressure were evident immediately post implantation. The calculated cardiac index had increased from 1.0 L/min/m2 at baseline to 2.7 L/min/m2 following TMVI. The patient was transferred to the ward on the second postoperative day for further convalescence and rehabilitation. He had a rapid recovery, and was discharged four days post implant. At two-month follow up, the patient described symptomatic improvement. Echocardiography documented the absence of mitral regurgitation with no change in LV function.
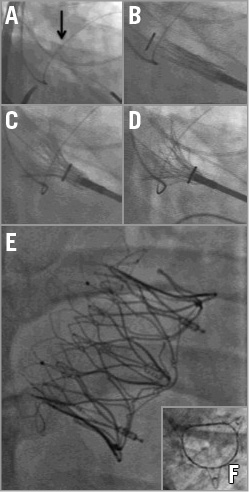
Figure 3. Transapical mitral valve implantation. A) A hydrophilic wire is inserted through the jugular vein into the coronary sinus (arrow) in order to assist in mitral valve plane visualisation and device positioning in fluoroscopy. B) & C) The delivery system is inserted into the left ventricle and across the mitral valve into the left atrium. The atrial skirt is then released. D) The ventricular anchors are released with capture of the anterior and posterior native mitral leaflets. E) A long-axis fluoroscopic image of the device frame. F) A short-axis image showing the D-shaped morphology of the device orifice.
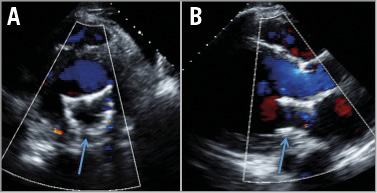
Figure 4. Post TMVI echocardiographic examination showing only trivial mitral valve regurgitation. A) Short axis. B) Long axis.
Discussion
SAFETY VS. EFFICACY OF MITRACLIP IMPLANTATION
The anatomy of the mitral valve is complex28,29. The valve and related structures include the annulus, valvular leaflets, chordae tendineae, and papillary muscles, and its function is closely related to the left ventricle morphology and contractility. Treatment of a single anatomic component may be ineffective. The MitraClip is the most widely utilised transcatheter device in patients with MR15. Repositioning before clip release is feasible, and multiple clips can be implanted if necessary. Although MitraClip implantation may be a safe and effective alternative to surgery in carefully selected high-risk patients with severe MR, its long-term durability in the absence of concomitant mitral annuloplasty is controversial30.
The MitraClip device was studied in the EVEREST II randomised trial, and in numerous registries, comparing it with conventional surgery16-18,31-34. MitraClip implantation is a relatively safe procedure. A recent analysis of 628 MitraClip implantations showed infrequent cardiac tamponade (1.1%) and stroke (0.2%). In addition, vascular damage and profuse bleeding requiring multiple transfusions were rare (1.1% and 0.7%, respectively)18. However, the efficacy of MR reduction post MitraClip implantation is not high. In the EVEREST II randomised trial, the primary endpoint of survival free from both surgery and significant MR at 12 months was lower with MitraClip compared to surgical valve replacement16,17.
Patients having significant MR post MitraClip implantation are challenging. These patients could be treated by conventional surgery35. However, MitraClip implantation excludes the potentially favourable approach of TMVI, the only mechanical approach for replacing the valve in patients considered inoperable. Fortunately, as shown in the current report, operators can assess the efficacy of MitraClip implantation during the procedure and remove the device if sustained efficacy does not seem probable and the patient is anatomically suitable for TMVI.
TRANSCATHETER MITRAL VALVE IMPLANTATION
In conventional surgery, valve replacement results in a more effective and durable relief of MR than repair, especially in secondary MR36. It is anticipated that TMVI will result in an effective, less invasive, approach for patients with severe MR. Clinical data in these procedures are currently limited. Transcatheter technologies aiming to replace the mitral valve are more difficult to develop than those for the aortic valve. This complexity includes the non-planar D-shaped annulus, lack of a fibrous annular structure, variability of leaflet and subvalvular apparatus anatomy, and proximity to the left ventricular outflow tract, circumflex coronary artery, and coronary sinus28,29. In addition, almost all of these procedures are performed utilising the transapical approach. It is already known that post apical procedure there is occasionally impairment in ventricular function37,38. This further deterioration, even if only transient, is clearly not desired in patients with functional MR who have significant ventricular dysfunction. We can speculate that in the future TMVI may probably be approached using an antegrade transseptal delivery.
Conclusions
In selected centres, utilising both the MitraClip and TMVI, the approach of “MitraClip first” could be considered. In this approach, a high-risk patient with severe MR could be treated with the widely studied and relatively safe MitraClip (when anatomical criteria are met); in case of limited efficacy, the first clip will not be released and the patient will be considered for the newly developed transapical TMVI. Assessment for TMVI and patient consent should be approached at an early stage.
| Impact on daily practice The clinical approach towards high-risk patients with significant MR may change in the next few years. Robust clinical data regarding TMVI are still lacking. The most important clinical implication to be derived from the present report is that, since a fully implanted and released MitraClip will exclude a patient from TMVI, if a patient is considered inoperable and during MitraClip implantation there seems to be very limited efficacy, the clip should not be released and the patient should be considered for TMVI. The clinical dilemma is between adding other clips or removing the first clip and attempting TMVI. We can expect that the TMVI option will probably place increased emphasis on the importance of having evident efficacy of first clip implantation during MitraClip procedures. |
Conflict of interest statement
A. Cheung has received consultation fees from Neovasc Inc. J. Leipsic has received consultation fees from Neovasc Inc. S. Banai is a medical director of Neovasc Inc. The other authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Significant mitral regurgitation post MitraClip implantation. Several attempts to clip A2 and P2 segments did not reduce mitral regurgitation severity. It was apparent that additional clips would not be effective and the clip was removed.
Moving image 2. A sheathless 32 Fr catheter is used for transapical delivery of the Tiara system.
Moving image 3. Release of the Tiara atrial skirt.
Moving image 4. The ventricular anchoring mechanism is released with capture of the anterior and posterior native mitral leaflets.
Moving image 5. Echocardiography following mitral valve implantation shows only trivial mitral valve regurgitation.
Moving image 6. Three-dimensional transoesophageal echocardiography showing the D-shaped Tiara device in the mitral valve orifice.
Supplementary data
To read the full content of this article, please download the PDF.
Significant mitral regurgitation post MitraClip implantation. Several attempts to clip A2 and P2 segments did not reduce mitral regurgitation severity. It was apparent that additional clips would not be effective and the clip was removed.
A sheathless 32 Fr catheter is used for transapical delivery of the Tiara system.
Release of the Tiara atrial skirt.
The ventricular anchoring mechanism is released with capture of the anterior and posterior native mitral leaflets.
Echocardiography following mitral valve implantation shows only trivial mitral valve regurgitation.
Three-dimensional transoesophageal echocardiography showing the D-shaped Tiara device in the mitral valve orifice.

