Introduction
Mitral valve disease is a common and undertreated condition that can result in congestive heart failure and cardiovascular death. Transcatheter mitral valve repair and replacement therapies are being employed to treat patients with severe MR who are deemed too risky for conventional surgery.
Neovasc Tiara transapical mitral valve implantation (TAMI) system
The Tiara™ TAMI system (Neovasc Inc., Richmond, BC, Canada) is a novel, catheter-based system for the treatment of mitral regurgitation (MR). Extensive preclinical and cadaveric studies have been conducted and the results have been published.
Tiara prosthesis
The Tiara bioprosthesis is based on a nitinol self-expanding platform. The frame is saddle-shaped to mirror the native mitral annular anatomy. The three-dimensional, asymmetric atrial skirt provides secure seating of the prosthesis onto the atrial side of the MV annulus. Axial anchoring of the device is provided by three ventricular tabs, two anteriorly and one posteriorly. The anterior tabs anchor onto the aortomitral fibrous trigone and the posterior tab onto the posterior shelf of the annulus. Along with radial expansion of the device, the Tiara can be firmly secured without the risk of migration and paravalvular leakage (PVL). Three bovine pericardial leaflets provide valve function, a large and non-obstructive orifice. To accommodate various annular dimensions, two valve sizes (35 and 40 mm) are currently available (Figure 1).
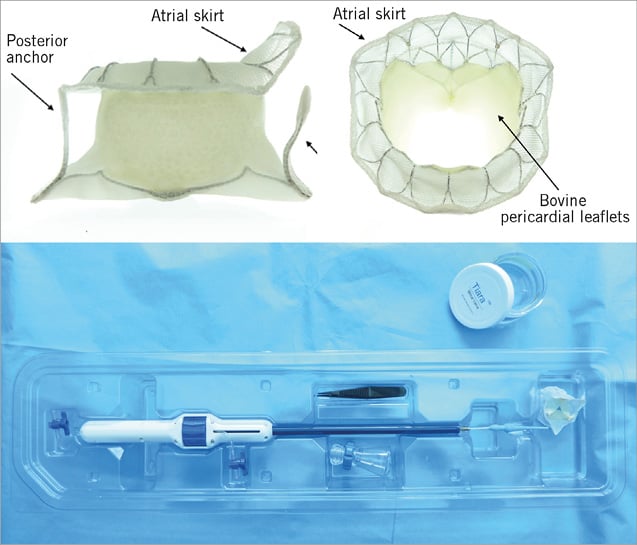
Figure 1. The Neovasc Tiara TAMI system.
Delivery system
The sheathless delivery catheter comprises a self-dilating tip with a turn knob mechanism. The self-dilating tip allows an atraumatic entry into the left ventricle (LV). The diameter of the system is 32 Fr for the 35 mm valve and 36 Fr for the larger device. Resheathing, repositioning and retrieval of the prosthesis are possible until the final stage of the deployment.
Patient screening
Extensive workup including basic investigations, coronary angiography, echocardiography and cardiac CT are performed to assess patient eligibility. Patients with LVEF <20%, severe RV dysfunction, or irreversible pulmonary hypertension are excluded. Anatomical suitability is mainly assessed by echocardiography and cardiac CT. Table 1 provides the mitral annular dimensions for the current 35 and 40 mm Tiara valves. In addition to annular dimensions, cardiac CT provides invaluable information for determining other anatomical details, such as LVOT dimension, angle and the anatomical fit of the Tiara device. Common patient exclusions include poor LV function, excessive mitral annular dimensions, absence of posterior shelf and high risk of LVOT obstruction.

Implant procedure
Tiara implants are performed in a hybrid operating room with general anaesthesia without the aid of cardiopulmonary bypass. The involvement of a multidisciplinary team of an interventional cardiologist, cardiac surgeon, cardiac imager and echocardiographer is crucial to implantation success. Pre-procedural cardiac CT imaging provides invaluable information regarding incision site, LV access and implant projections.
A typical small, 4 cm left mini anterior thoracotomy is performed to gain access to the LV. Placement of two octagonal pledgeted sutures provides haemostasis of the LV apex. LV access is achieved by needle puncture, followed by the introduction of a 0.035-inch J wire across the mitral valve into the left atrium (LA) under transoesophageal echocardiographic (TEE) and fluoroscopic guidance. It is then exchanged for a 0.035-inch Amplatz Extra-Stiff™ wire (Boston Scientific, Marlborough, MA, USA). The Tiara TAMI delivery system is introduced and advanced across the MV into the LA, followed by the unsheathing of the atrial portion of the Tiara. Anatomical orientation of the saddle-shaped Tiara to the native MV is then conducted with TEE guidance. Gentle traction towards the LV allows seating of the atrial skirt onto the atrial MV annulus. The ventricular portion and the anchoring tabs are then released with further unsheathing of the device. Resheathing, repositioning and retrieval of the device can be safely performed prior to the release of the ventricular skirt. After the release of the Tiara, the delivery system is resheathed and removed from the LV apex. Figure 2 demonstrates post-Tiara implantation in a patient with an aortic bioprosthesis in situ. Detailed post-implant TEE demonstrates a well-functioning Tiara, with no MR, PVL or left ventricular outflow tract (LVOT) obstruction (Moving image 1).
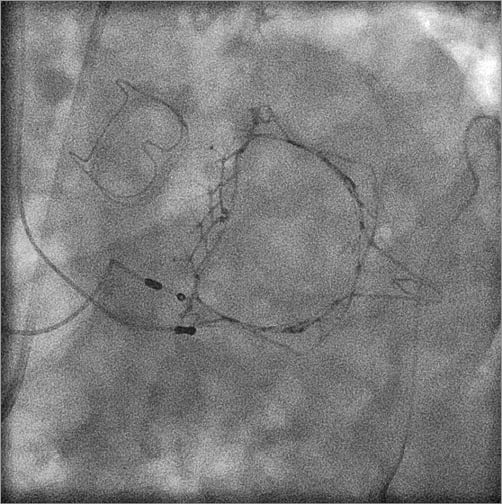
Figure 2. Post Tiara implantation in a patient with previous aortic valve replacement.
First-in-human implantation
The first clinical Tiara implantation was carried out in January 2014 at St. Paul’s Hosptial, Vancouver, Canada. A non-surgical, frail 73-year-old man with severe ischaemic cardiomyopathy, LVEF of 15% and severe functional MR was treated under Canadian compassionate protocol. His calculated STS risk of mortality for mitral valve replacement (MVR) was 47.7%, the logistic EuroSCORE II was 24.7%, and his comorbidities included chronic renal insufficiency requiring intermittent haemodialysis and severely impaired pulmonary function due to pulmonary fibrosis. He underwent an uneventful implantation of the Tiara. Despite complete elimination of the MR and a well-functioning prosthetic mitral valve, the patient was readmitted with recurrent end-stage heart and renal failure and was referred for palliation.
Successful implantations have been conducted under compassionate access programmes in Canada (St. Paul’s Hospital, Vancouver), in Italy (San Raffaelle Hospital, Milan) and in Germany (Universitäres Herzzentrum, Universitätsklinikum Eppendorf, Hamburg). Two patients have passed their two-year anniversary and are clinically well. Echocardiographic studies demonstrate well-functioning prostheses with no signs of thromboembolism or PVL, and with low transvalvular gradient. Cardiac CTs show stable valve fixation and no structural deterioration.
TIARA-I Trial
The TIARA-I Early Feasibility Trial is a multinational, multicentre trial being conducted in Canada, USA and Belgium. The primary objective is to assess the 30-day safety outcomes of the Tiara TAMI system and implantation procedure in high-risk surgical patients suffering from severe MR. Secondary objectives include device performance, adverse events and clinical outcomes up to one year. First enrolment and successul implantation were carried out by the team at ZNA Middelheim, Antwerp, Belgium, led by Dr Stefan Verheye in December 2014. The TIARA-I study is actively enrolling at the present time.
Conclusions
Mitral regurgitation is a common valvular pathology and patients are being left untreated. The Tiara TAMI is a catheter-based mitral replacement technology specifically designed to treat mitral regurgitation. The first-in-human experience has demonstrated technical feasiblity and saftey of the Tiara TAMI system. Complete abrogation of MR, secure device anchoring, low transvalvular and LVOT gradient and the absence of significant PVL were achieved. Ongoing compassionate implantations, follow-up and the TIARA-I clinical trial will further provide important clinical data and proof of device feasiblity and performance.
Conflict of interest statement
A. Cheung is a consultant and proctor for Neovasc Inc. S. Banai is chief medical officer with Neovasc Inc.
Supplementary data
Moving image 1 (A & B). Well-functioning Tiara, no MR, PVL or LVOT obstruction.
Supplementary data
To read the full content of this article, please download the PDF.
Well-functioning Tiara, no MR, PVL or LVOT obstruction.
Moving image 1b

