Introduction
The transcatheter mitral valve replacement (TVMR) technique represents a promising approach for treating mitral valve regurgitation in patients at increased risk of perioperative mortality. At present, several transcatheter mitral valve systems are under preclinical and clinical investigation. Each of them has specific features which have the aim of ensuring predictable delivery, an efficient anchoring and sealing to the mitral annulus, whilst preserving surrounding cardiac structures and the patency of the left ventricular outflow tract. The CardiAQ transcatheter mitral valve (Edwards Lifesciences, Irvine, CA, USA) has already been tested in humans with promising outcomes. The key feature of this TMVR technology rests on its capability to be delivered through a transseptal approach in addition to a transapical approach.
Device overview
The current CardiAQ TMV consists of a self-expanding nitinol frame, which has three leaflets of bovine pericardial tissue and a fabric intra-annular sealing skirt (Figure 1). The attachment mechanism comprises left ventricular anchors that engage the native mitral leaflets and annulus, and preserve the subvalvular apparatus. The symmetrical design requires no rotational alignment, and the valve is delivered through a 33 Fr transseptal or transapical delivery catheter.
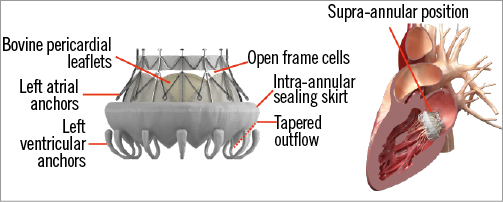
Figure 1. The CardiAQ transcatheter mitral valve.
Procedure overview
TRANSAPICAL APPROACH
The procedure is performed under general anaesthesia, using fluoroscopic and transoesophageal echo guidance (Figure 2, lower panels). The technique for valve delivery has undergone several refinements in recent years. The CardiAQ valve was specifically designed for transseptal delivery, and the first-in-human transseptal implantation was performed at Rigshospitalet in Copenhagen, Denmark, on 12 June 2012. Subsequent implants with the second-generation CardiAQ prosthesis were accomplished using the transapical approach, while the transseptal delivery system underwent further development. Transapical access provides a close and direct access to the mitral valve, and device alignment is generally easily achieved. Accurate prosthesis positioning and deployment were obtained, proving the feasibility of the procedure and the capability of the CardiAQ prosthesis to remain well anchored within the native mitral valve and provide a good seal. Since then, fully transseptal deployments have also been performed with the second-generation CardiAQ valve.
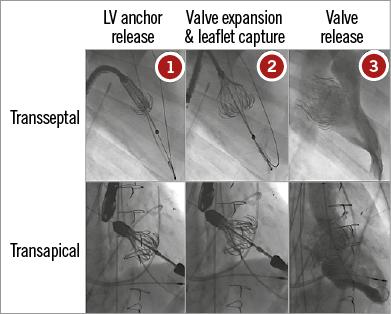
Figure 2. Procedural steps of CardiAQ transcatheter mitral valve deployment through the transseptal route (upper panels) and the transapical route (lower panels).
Courtesy of Edwards Lifesciences
TRANSSEPTAL PROCEDURE
The procedure is performed under general anaesthesia (Figure 2, upper panels). Two femoral vein access sites are obtained, one for transseptal puncture and the delivery system and one for a steering snare to help guide the system into the left ventricle. In addition, two arterial femoral access sites are also used, one for a pigtail for left ventricle angiograms, and one for an additional snare that allows for the creation of the arteriovenous loop (the wire, advanced through the septum, left atrium and left ventricle, is snared in the descending aorta and externalised from the femoral artery). The first transseptal procedure in 2012 was performed using a single septal puncture: the snaring of the wire that hosts the delivery catheter system (DCS) was initially performed in the iliofemoral vein, and the DCS with the snare looped around crossed the interatrial septum through a single puncture site. More recently, Ussia et al described a modified technique, which foresees a separate transseptal access of the DCS and the snaring system. With this solution, the DCS capsule seems to be more easily steerable from the left atrium to the left ventricle, reducing the trauma of the interatrial septum. Before advancing the DCS with the prosthesis loaded into its distal portion, a 20 to 25 mm inflated balloon is tracked on the wire from the left atrium to the left ventricle outflow tract to ensure that the wire is not caught in the mitral apparatus. The valve is then advanced through the femoral vein to the level of the mitral valve.
The landing zone is between the mitral plane and the tip of the papillary muscles. This is determined by transoesophageal echocardiography and a left ventriculogram. By manipulating the arteriovenous loop and the delivery system, the prosthesis is positioned perpendicular to the mitral annular plane. A staged deployment allows exposure of the ventricular anchors and careful positioning to capture the posterior and anterior mitral leaflets. The final position is checked with echocardiography and the prosthesis is then released.
Clinical data
The first-in-human implantation of the first-generation CardiAQ bioprosthesis was performed in June 2012. From May 2014 to June 2016, there have been around 15 implants with the second-generation CardiAQ bioprosthesis, using both the transapical and the transseptal approach. These early implants showed the feasibility, safety and efficacy of this procedure. Twelve patients with severe mitral regurgitation have beeen treated under compassionate use as of November 2015 (11 patients with the current-generation valve). The aetiology of mitral regurgitation was functional in 64% and degenerative in 36% of cases. Technical success (successful delivery, deployment and retrieval of the delivery system) was achieved in 82% of patients. Two procedure-related deaths were described, one interaction with the mechanical aortic valve and one malpositioning due to sub-leaflet calcification. At 30 days, three additional non-valve-related deaths were reported (all patients had good valve function). The CardiAQ prosthesis has been implanted under compassionate use. The US early feasibility study is currently ongoing and the CE mark trial is expected to start in late 2016.
Conflict of interest statement
M. Barbanti is a consultant for Edwards Lifesciences. C. Tamburino has received speaker honoraria from St. Jude Medical and Medtronic Inc.

