
Abstract
Aims: Transcatheter mitral valve implantation for mitral valve regurgitation is in the very early phase of development because of challenging anatomy and device dimensions. We describe the procedure of a transfemoral-transseptal implantation of the second-generation CardiAQ™ mitral valve bioprosthesis and 30-day follow-up.
Methods and results: The procedure was performed percutaneously, without any left extracorporeal circulatory support. The patient had severe mitral regurgitation with severely depressed ventricular function and other comorbidities. The patient was deemed extreme high risk for conventional cardiac surgery by a multidisciplinary team. The main procedural steps were the creation of an arteriovenous loop with an exchange nitinol wire, and the use of a customised “steerable snare system” to facilitate the catheter delivery system into the mitral annulus. Transoesophageal echocardiography and fluoroscopy were utilised for device positioning and deployment. The mitral valve prosthesis was implanted with mild mitral regurgitation. The postoperative course was uneventful and at 30-day follow-up the patient is in NYHA Class I, with good function of the mitral valve bioprosthesis.
Conclusions: This procedure shows that percutaneous transfemoral transcatheter mitral valve implantation is feasible, safe and successful. Further experience is needed to render this procedure clinically available.
Introduction
Transcatheter mitral valve implantation (TMVI) for the treatment of mitral valve regurgitation is in the very early phases of development. The TMVI experience with a dedicated bioprosthesis is limited to compassionate use in very sick patients1. Transfemoral implantation is technically challenging with the devices currently available. One patient was treated in 2012 with the support of extracorporeal circulation with the first-generation CardiAQ mitral valve (CardiAQ™ Valve Technologies, Inc., Irvine, CA, USA)2. Since then, only transapical procedures have been performed3. We describe the first totally percutaneous transfemoral-transseptal mitral valve implantation with the second-generation CardiAQ™ Transcatheter Mitral Valve Implantation system (CardiAQ™ Valve Technologies, Inc.).
Device description
The second-generation CardiAQ™ TMVI system consists of a transcatheter mitral valve bioprosthesis, and a transapical or transfemoral catheter delivery system (CDS). The CardiAQ bioprosthesis (Figure 1) is a trileaflet bovine pericardial valve housed in a self-expanding nitinol frame. The frame has two sets of opposing anchors, which serve to secure the device in the mitral annulus. The left ventricular anchors are designed to engage the mitral valve leaflets and subvalvular apparatus. The frame is covered by a polyester fabric skirt to reduce paraprosthetic leaks. The transfemoral delivery catheter has three different sections. The distal section has a diameter of 33 Fr and houses the loaded valve; it tapers in a second soft section of 22 Fr diameter (polytetrafluoroethylene [PTFE] coated) and continues in the rigid shaft of the catheter. The handle controls the exposition and release of the valve.

Figure 1. CardiAQ™ Transcatheter Mitral Valve Implantation system.
Patient and procedure description
The patient was a 72-year-old man with congestive heart failure secondary to post-ischaemic cardiomyopathy, NYHA functional Class III. His comorbidities were chronic atrial fibrillation, triple coronary bypass surgery, history of sustained ventricular and factor VII deficiency. A multidisciplinary team considered him to be extreme high risk for surgical mitral valve replacement. The transoesophageal echocardiogram (TEE) showed a severely dilated left ventricle (LV), posterior and inferior wall akinesia with severe impairment of the LV function (left ventricular ejection fraction [LVEF] 30%), and severe mitral regurgitation secondary to tethering of both leaflets with extreme retraction of the posterior leaflet (Figure 2).
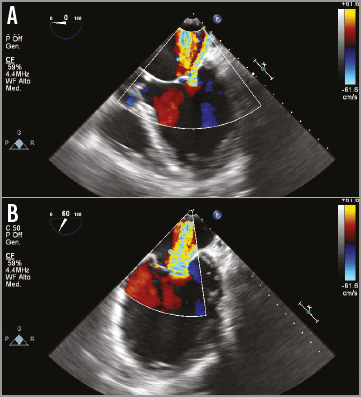
Figure 2. Preprocedural transoesophageal echocardiography. A) Severe mitral regurgitation with tethering of the mitral posterior leaflet in the four-chamber view. B) Intercommisural view.
The multiplanar reconstruction of the cardiac computed tomography was used to measure the intercommissural diameter and the anteroposterior diameter of the mitral annulus, the height of the left atrium (LA), the LV outflow tract dimension and the angle between the mitral annulus plane and the aorta. Anatomical exclusion criteria were: mitral annulus larger than 40 mm, very acute mitro-aortic angle; short superoinferior left atrial dimensions; severe calcification of the mitral valve apparatus. Hence, the heart anatomy was deemed suitable for implantation of a CardiAQ mitral bioprosthesis. Ethical committee approval for compassionate use was obtained.
The procedure was performed in a cardiac catheterisation laboratory with cardiac surgery back-up. Both femoral arteries and veins were utilised during the procedure. The right femoral vein (FV) was accessed after insertion of a Perclose ProGlide® Suture-Mediated Closure System (Abbott Vascular, Abbott Park, IL, USA). We performed a first transseptal puncture using a standard 8 Fr transseptal Mullins™ sheath and Brockenbrough® needle (St. Jude Medical, St. Paul, MN, USA). The puncture site was located in the posterior part of the interatrial septum (IAS) about 5 cm above the mitral valve (MV) in order to obtain the best alignment possible of the CDS capsule inside the native MV annulus (Figure 3).
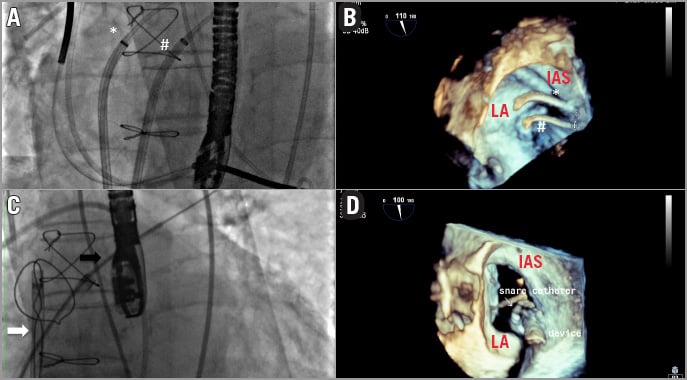
Figure 3. Transseptal puncture locations. A) The two transseptal introducers are shown: the superior puncture (*) is used for the delivery catheter insertion, and the snare system is placed in the inferior puncture (#). B) The relationship between the two transseptal accesses is displayed using 3D echocardiography. C) The “snare system” capturing the 12 Fr Mullins sheath (black arrow). D) 3D echocardiogram shows the relationship between the snare and the delivery system. LA: left atrium; IAS: interatrial septum
This access was predilated with a standard peripheral 10 mm balloon, to allow smooth entry of the 33 Fr CDS and, thereafter, a long 12 Fr Mullins Performer™ Guiding Sheath (Cook Medical, Limerick, Ireland) was placed in the left atrium. A second puncture was performed 1 cm below the first one and a second 8 Fr steerable catheter (Destino™; Oscor Inc, Palm Harbor, FL, USA) was positioned in this location from the left femoral vein into the LA (Figure 3). A snare of 40 mm diameter and its introducer sheath of 6 Fr (pfm medical ag, Cologne, Germany) were placed inside the steerable catheter. The wire coming from the first superior transseptal access was caught by the snare coming from the second inferior transseptal access inside the left upper pulmonary vein; the “snare system” was prolapsed into the LA, and a balloon-tipped Swan-Ganz (SG) catheter (Medtronic Inc., Minneapolis, MN, USA) was inserted in the LA coming from the superior transseptal access inside the snare, and was advanced across the MV annulus into the LV and into the ascending aorta. An exchange wire was inserted into the descending aorta through the SG and snared (AndraSnare® AS-25 set; Andramed GmbH, Reutlingen, Germany) from the right femoral artery (FA). The SG catheter was exchanged for a 46 mm Reliant® balloon catheter (Medtronic Inc.) which was gently advanced from the LA to the aorta to check that the wire was not passing through the mitral chordae (Figure 4). The standard wire was changed for the customised nitinol wire and the arterial end was snared and extracted from the left FA to create an arteriovenous loop (RF vein-inferior vena cava-RA-LA-LV-aorta-RF artery) intended to be used for antegrade delivery of the prosthesis. After the 12 Fr Mullins sheath was removed, the CDS was inserted under fluoroscopy control, from the right FA into the LA, where the capsule was snared. To advance the system across the mitral valve, adequate tension was applied to the arterial and venous sections of the wire loop so that the device could be advanced in the mitral annulus while applying traction with the snare (Figure 5).
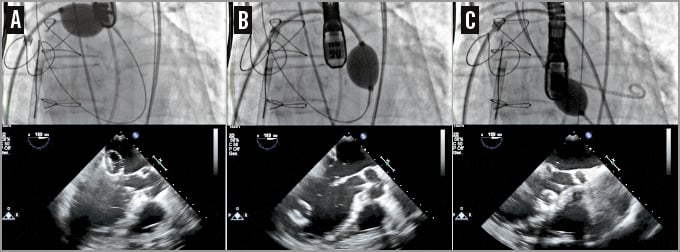
Figure 4. Checking the wire pathway. A balloon catheter pushed over the wire across the mitral valve from the left atrium (A), the left ventricle (B) and the left ventricular outflow tract (C). TEE follows the balloon trajectory and shows if the wire is inside the chordae tendineae.
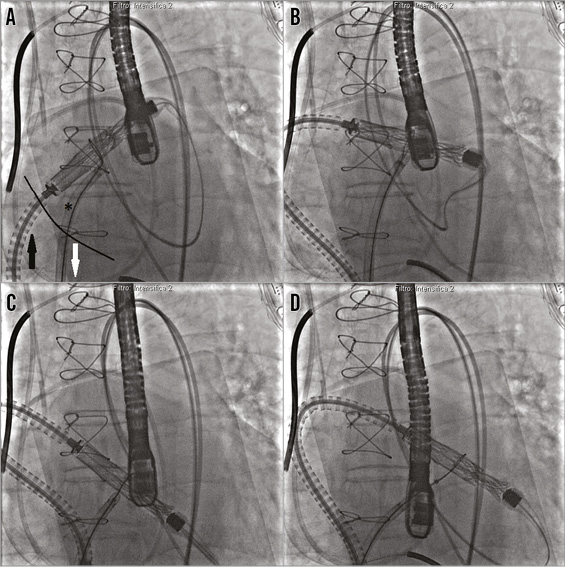
Figure 5. Capsule navigation across the mitral valve. A) The capsule is snared by the snare system (*) positioned in the left atrium through the inferior septal puncture. The capsule is pulled towards the inferior vena cava (white arrow) while the catheter delivery system is pushed across the superior transseptal access (black arrow). B) and C) The capsule is across the mitral valve. D) The snare is released and pulled away from the capsule.
Next, the steps of valve alignment were followed using the wire loop, as recommended by the manufacturer based on their experimental experience in animal models. Under the guidance of 2D and 3D TEE and X-plane views, we found the optimal depth for the valve and checked the centrality. When the anchors were at the level of the mitral leaflet rims, we slowly started to expose the LV part (Moving image 1). Once the anchors were exposed, the snare was retracted and, manipulating the arteriovenous nitinol wire, we obtained the best alignment possible. We checked that all the anchors were engaging both leaflets and, once the LV anchors captured the MV leaflets, the LA anchors were exposed. The final position was checked with echocardiography and the bioprosthesis was released (Figure 6). The capsule and nose cone were closed and the CDS was removed from the RF vein. The final echocardiogram and LV angiogram confirmed the stability of the prosthesis in the mitral annular position, without interference with the surrounding structures (Moving image 2); trivial posterior paravalvular regurgitation was detected (Figure 7).
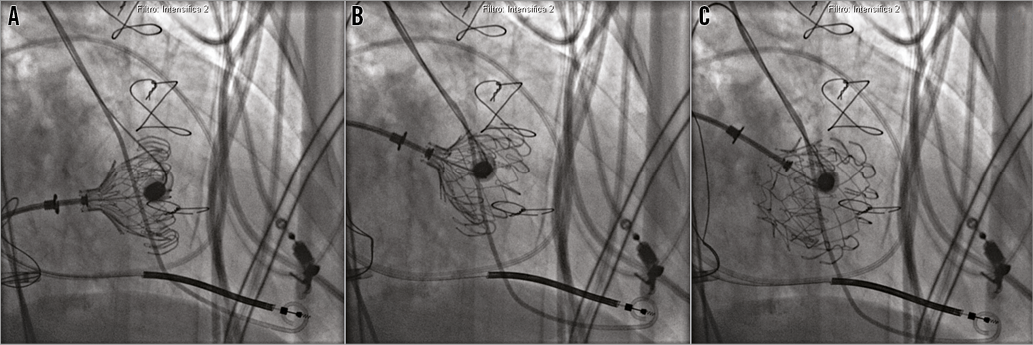
Figure 6. Deployment of the CardiAQ prosthesis. A) After the exposition of the left ventricular anchors, the left atrial anchors are exposed. B) With manipulation of the nitinol wire loop and the delivery system, the prosthesis alignment is optimised. C) Valve released after final echocardiographic and angiographic check.
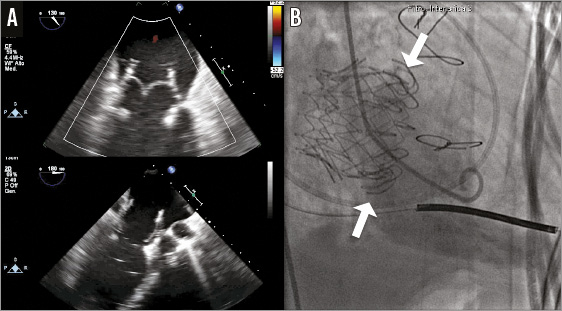
Figure 7. Echocardiographic and angiographic control after bioprosthesis release. A) Transoesophageal echocardiogram confirms the bioprosthesis function. B) Left ventriculography shows the stable positioning of the mitral valve bioprosthesis and the left ventricular anchors under the native mitral annulus (white arrows).
After removal of the CDS, a residual iatrogenic IAS tear with left to right shunt was observed, followed by closure with a 35 mm patent foramen ovale occluder device (Figulla® PFO occluder; Occlutech GmbH, Jena, Germany) (Figure 8). The right FV was closed with the pre-implanted suture lines and the haemostasis was completed with “figure of eight” sutures; all the other vascular accesses were closed by manual compression. The patient was extubated in the catheterisation laboratory at the end of the procedure. During all the procedural steps, the patient was haemodynamically stable, without the need for any left ventricular mechanical support. The postoperative course was uneventful. The patient stayed three days in the intensive coronary care unit and was discharged seven days later with low molecular weight heparin (LMWH) and acetylsalicylic acid (ASA). At one-month follow-up, the patient is NYHA Class I, the mitral prosthesis is functioning well without any structural deterioration, and post-procedural paravalvular regurgitation is absent.
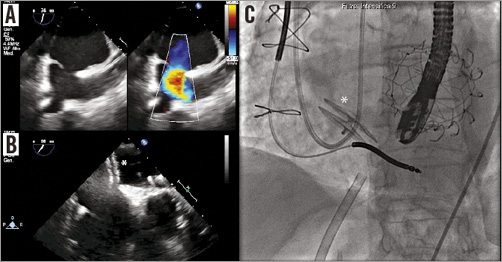
Figure 8. Residual interatrial communication closure of the transseptal access. A) Left to right shunt across an iatrogenic interatrial communication. B) Closure of the interatrial communication with a 35 mm PFO occluder (*). C) Correct position of the PFO occluder device on fluoroscopy.
Discussion
We described the first procedure ever performed of percutaneous transfemoral-transseptal TMVI with the second-generation CardiAQ system with a 30-day follow-up. The first transfemoral TMVI was carried out in 2012 by Sondergaard2 with the first-generation CardiAQ valve; the patient had a lethal complication at 68 hours. Since then, a few patients have been treated using the transapical access because of better control of the CDS. The procedure proved to be safe, feasible and successful. We found the use of a “snare system” across a separate transseptal access extremely useful. With this solution, the CDS capsule was easily oriented from the LA to the LV, reducing the trauma of the IAS. The telescope system of the 6 Fr snare introducer inside the 8 Fr steerable guiding catheter allowed a controlled manipulation, avoiding entanglement of the bioprosthesis.
The use of an SG catheter for the creation of the arteriovenous loop simplified this manoeuvre, avoiding guidewire entrapment in the chordae tendineae, which can occur with a standard diagnostic catheter. The patient showed stability of all the haemodynamic parameters, despite the 33 Fr CDS capsule inside the native mitral valve and arteriovenous wire manipulations, without the need of any mechanical LV support. TEE played a fundamental role in all procedural steps. The imaging quality of bioprosthesis relationship with all the cardiac structures was high, and the capture of native mitral leaflets by the device LV anchors was clear and reliable. The valve prosthesis started to function immediately after release, without residual gradient. There was trivial paravalvular regurgitation in correspondence of the lateral commissure which disappeared at 30-day follow-up control. Tearing of the interatrial septum was expected, because of the manipulation of the CDS and the snare system. The left to right shunt did not modify the haemodynamics and was easily closed with a PFO occluder device.
Some authors have expressed concern about mitral annulus deformation by the circular bioprosthesis with structural and functional consequences1. The CardiAQ device lodged perfectly inside the D-shaped native mitral valve annulus (Figure 9, Moving image 3), and we did not observe any interference of the surrounding structures (the left circumflex artery, the coronary sinus, the electric conduction system, the mitro-aortic continuity) or LVOT obstruction. Postoperative management was uneventful: it did not require any inotropic support, and the standard therapy was maintained with ACE inhibitors, beta-blockers and lower doses of furosemide. The anticoagulation regimen consisted of LMWH and ASA, as recommended by the haematologist because of factor VII deficiency.
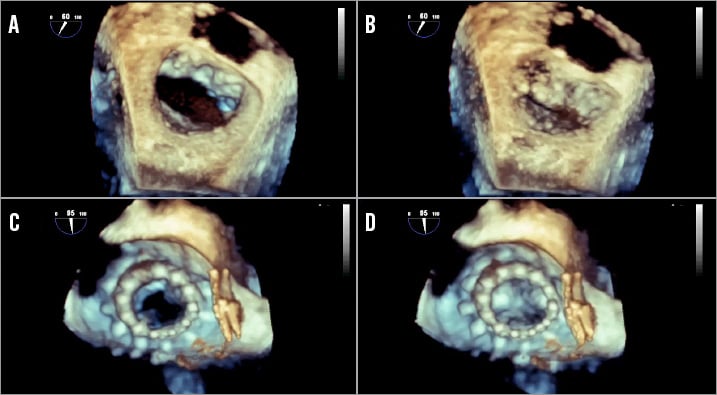
Figure 9. 3D reconstruction of the mitral valve in surgical view. The native mitral valve in the diastolic (A) and systolic (B) phase. The CardiAQ device after the implantation in the diastolic (C) and systolic (D) phase.
Conclusion
In conclusion, our very first experience with the totally percutaneous use of the CardiAQ Transcatheter Mitral Valve Implantation system showed the feasibility, safety and efficacy of this procedure at 30 days. In particular, the patient showed an unexpected haemodynamic stability, and the echocardiographic imaging was very precise in displaying the anatomic relationship of the device. There is scope to implement this device and the procedure; however, a clinical trial is needed to show the safety and durability of the prosthesis.
| Impact on daily practice This paper describes the first truly percutaneous mitral valve implantation using the latest generation of a transcatheter mitral valve bioprosthesis. The feasibility of the procedure, and the procedural and clinical success, will provide a strong impulse to this technology, which will impact deeply on the future treatment of mitral valve regurgitation, once the device has been ameliorated and the interventional technique has been standardised. |
Conflict of interest statement
G.P. Ussia and L. Sondergaard are consultants for CardiAQ Valve Technologies Inc. A. Quadri is founder, chairman and CMO of CardiAQ Valve Technologies Inc. The other authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Right anterior oblique view.
Alignment of the mitral valve bioprosthesis before the release.
Moving image 2. 2D echocardiogram with colour Doppler after CardiAQ mitral valve release.
Moving image 3. 3D reconstruction of the CardiAQ mitral valve and PFO occluder.

