Abstract
Mitral regurgitation (MR) is the second most common valvular heart disease in the western world with a prevalence in the USA alone of two to four million people. MR is age-dependent and hence the MR burden is expected to increase with the increase in life expectancy observed in Europe and the USA. Medical and surgical treatment has been the cornerstone of the treatment as untreated MR results in poor prognosis. However, only a fraction of patients are offered surgical treatment due to high risk. With the success of transcatheter aortic valve implantation (TAVI), many transcatheter options have emerged to provide a less invasive option for these patients. On the one hand, the repair options appear less invasive but tend to reduce the MR, while on the other hand the replacement options can eliminate the MR but could be more invasive. This article provides an overview of emerging transcatheter mitral valve replacement (TMVR) options currently available and summarises challenges in device design and patient selection and also early results.
Introduction
Mitral regurgitation (MR) is the most common valvular disorder and has a growing prevalence in the ageing population. It is either of degenerative (myxomatous degeneration of the leaflets and chordal structures leading to prolapse or flail leaflets), functional (due to left ventricular annular dilation, leaflet tethering, and malcoaptation), or mixed aetiology (Figure 1). MR is most often managed conservatively or surgically. An increasing number of percutaneous options for mitral valve repair and replacement are becoming available for patients at high surgical risk. Patient-specific factors, including frailty and comorbidities, in addition to anatomic considerations, are important to consider when determining which devices may be of use for mitral valve repair and replacement in these patients. In this article, we will focus on transcatheter mitral valve replacement (TMVR) techniques.
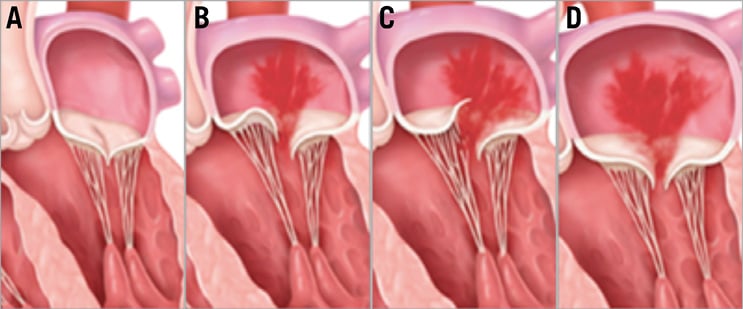
Figure 1. Types of mitral regurgitation. A) Normal mitral valve. B) Degenerative MR caused by mitral leaflet prolapse. C) Degenerative MR caused by flail leaflet. D) Functional MR caused by dilated ventricle and tethering of the mitral leaflets.
Mitral valve anatomy relevant to percutaneous techniques
It is important to understand the mitral anatomy and its relationship with the surrounding structures relevant to percutaneous techniques. The mitral valve is a complex apparatus that includes the annulus, the leaflets, the chordae, and the papillary muscles (PM). As the PM originate from the left ventricle (LV), the mitral apparatus plays a fundamental role in the structural and functional integrity of the LV. Therefore, disruption of the mitral-ventricular geometry could result in maladaptive remodelling and impaired LV performance1.
Mitral valve annulus. The mitral annulus is an oval, saddle-shaped structure, that provides the base for the anterior and posterior mitral valve leaflets. The anterior annulus (one third of the total circumference) is fibrous but the posterior annulus (two thirds of the circumference) is predominantly muscular and hence prone to dilatation (Figure 2A). The size of the annulus changes during different parts of the cardiac cycle with an estimated reduction of 25% of the diameter during systole2. The location of the annulus is variable in relation to adjacent vascular structures but can lie 1 cm below the coronary sinus and 2 cm below the circumflex artery. Furthermore, the length of contact between the coronary sinus and the posterior annulus varies and is relevant to therapies based on external annular compression (Figure 2B). The proximity of the posterior annulus to the circumflex artery is important, as variation in anatomy dictates the risk of injury to the circumflex artery (Figure 2B).
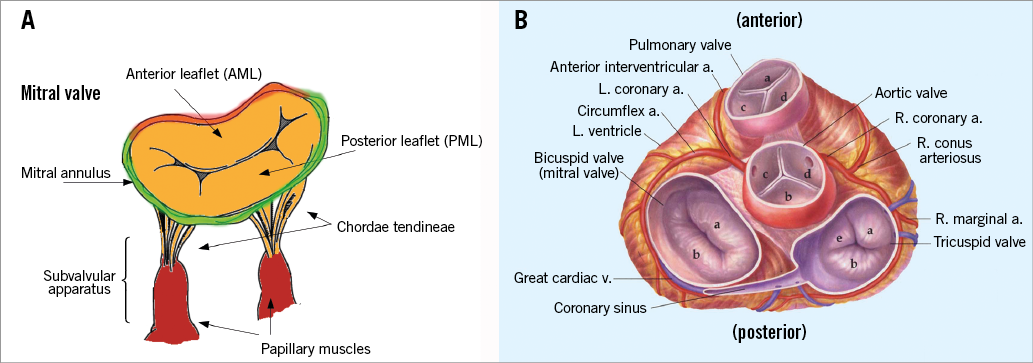
Figure 2. Mitral valve and anatomical relationship with surrounding structures. A) The mitral valve anatomy: the mitral valve is a complex structure, comprising the mitral valve annulus (anterior annulus - red colour; posterior - green colour), two leaflets, chordae tendineae and papillary muscles. B) End-on view of all four heart valves demonstrating the external relationship between the posterior mitral valve apparatus and the coronary sinus and circumflex coronary artery.
Leaflet and subvalvular apparatus. The mitral valve has two leaflets, a wider anterior mitral leaflet (AML) and a longer posterior mitral leaflet (PML). The leaflets are attached at their base to the mitral valve ring and they are attached with their free edges to the left ventricle via the subvalvular apparatus. Both leaflets receive chordae from both papillary muscles3 (Figure 2A). All TMVR devices interact with the leaflets and the subvalvular apparatus. Thus, pathologies of these structures, such as a flail or tethered leaflet, calcification of the leaflet, fused subvalvular apparatus, or abnormal papillary muscle morphology, may pose a challenge for the placement and secure deployment of TMVR devices. Usually, there are two papillary muscles – an anterolateral and a posteromedial PM. It must be recognised that these structures are dependent on adequate myocardial blood flow through the coronary arteries for optimal function. The anterolateral PM is often a single structure with dual blood supply from the left coronary artery, whereas the posteromedial PM is usually a multi-head structure with blood supply from only the right coronary artery4 (Figure 2A).
Left ventricular (LV) morphology. As the PM connect the LV wall with the mitral apparatus, any changes in the LV geometry can result in mitral valve dysfunction due either to leaflet tethering or to annular dilatation. Because most TMVR devices will project to a varying degree into the left ventricular cavity, any deformation of the LV geometry may affect device implantation and function. Because the majority of ventricles with MR are dilated due to volume overload, TMVR device design must take the degree of LV dilatation into account. LV geometry can also influence the optimum delivery angle and apical anchoring of devices, both representing important components of TMVR implantation5. Finally, the presence of a left ventricular septal bulge can limit the suitability of a patient for a particular type of TMVR.
Left ventricular outflow tract (LVOT). The AML is essentially a curtain, which divides blood flow between the LV inflow and outflow. During surgical mitral valve replacement, the AML is removed. This is not possible during TMVR. Hence, the AML will be held open by the TMVR and essentially wraps the TMVR device similar to a covered stent. The wrapping could, however, result in LVOT obstruction (LVOTO)6,7. Various factors, such as the size of the LV cavity, septal bulge, aortomitral annular angle, length and bulk of the AML, and finally length of the TMVR device in the LV will determine the presence and degree of LVOTO7,8 (Figure 3).
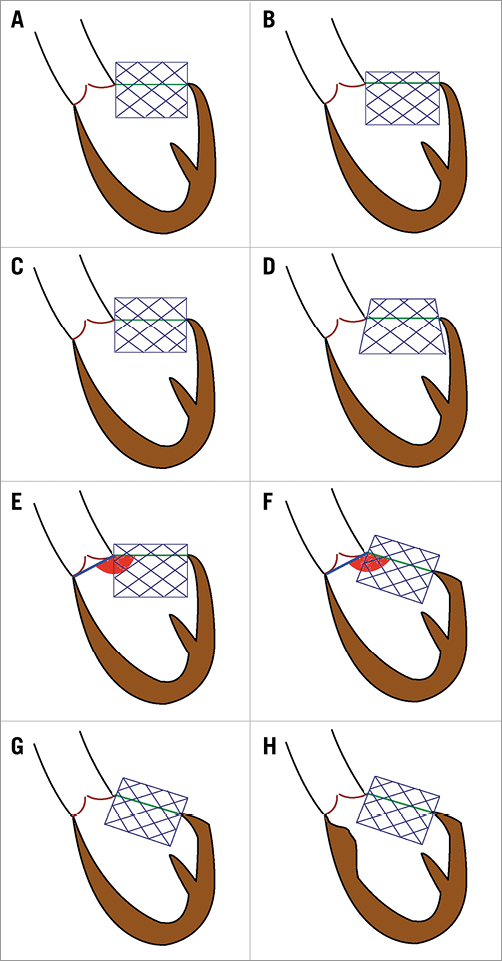
Figure 3. Anatomic factors which might be associated with the risk of LVOTO in TMVR. A) & B) Effect of depth of valve implant. C) & D) Effect of flaring of the device. E) & F) Aortomitral annular angle. G) & H) Extent of septal bulge. Reproduced with permission from Tang et al8.
Transcatheter mitral valve replacement (TMVR)
Current experience with transcatheter mitral valve repair is far more extensive than that with replacement. However, in some patients, mitral valve repair is not feasible or effective. TMVR has several advantages, compared with valve repair, as it can potentially eliminate MR, irrespective of the underlying pathology. TMVR also preserves the chordae and leaflets and hence helps to preserve left ventricular function9. As is the case in surgery, transcatheter techniques for repair and replacement are likely to be complementary.
DEVICE DESIGN
The “Ten Commandments” of an ideal TMVR design would be:
1. Ease of implantability
2. Reproducibility
3. Results in complete elimination of MR
4. No risk of LVOTO
5. No adverse effect on LV function
6. Addresses a wide range of sizes
7. Effective independent of the aetiology
8. Long-term durability
9. Non-thrombogenic
10. Does not result in haemolysis
First-in-man experience has now demonstrated feasibility and proof of principle for many devices but these early experiences have also revealed challenges10. When designing a TMVR device, there are numerous challenges, that can be grouped as follows:
– Anatomical: large and saddle-shaped annulus, varying morphology of leaflets depending on the pathology, size of the left ventricle and proximity to the LVOT. The risk of LVOTO is determined by various factors including LV size, septal bulge, aortomitral annular angle, length and bulk of the AML, and the length of the TMVR device.
– Physiological: higher closing pressures influencing stability, effects on blood flow in the atrium and LVOT, and thrombogenicity. Higher closing pressures also impact on durability. The risk of thrombosis and need for anticoagulation in patients with these devices are currently unknown.
– Challenges with device delivery: delivery systems and route of delivery.
Thus, the design of potential therapeutic devices is rather complex, particularly when compared with the development of TAVI devices.
DELIVERY SYSTEM
The larger nature of the device and delivery routes influence the construction of the delivery system. Designing a suitable delivery system that allows a safe route of delivery is a key factor affecting outcome. As the majority of TMVR devices are manufactured from a nitinol stent frame, most delivery systems are self-contained, i.e., the device is crimped within the delivery system. Some of the common features are:
A gradual, stepwise deployment of the device and partial or complete retrieval of the device within the sheath if the result is not satisfactory.
Access route defines the orientation of the device within the delivery system. Thus, a TMVR device delivered through a transapical (TA) approach will have the atrial portion towards the distal end of the delivery system.
As the device size is relatively large, most delivery systems will be larger than the current TAVI delivery systems, i.e., >30 Fr outer diameter (OD).
APPROACHES
The mitral valve can be accessed through multiple approaches, i.e., TA, transseptal (TS) or transatrial (TAt). The majority of TMVR implants to date have been performed through the TA approach, which reflects the ease of access to the mitral valve, size and manoeuvrability of the delivery system.
TA APPROACH (Figure 4A)
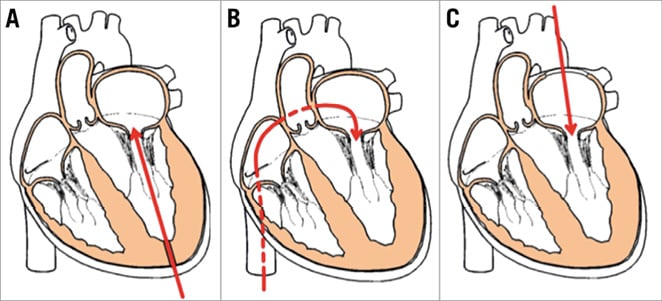
Figure 4. Common approaches for TMVR. A) Transapical. B) Transseptal. C) Transatrial.
The TA approach is similar to the TA approach in TAVI, but with some important differences. In contrast to TAVI, the site of the puncture needs to be accurately determined, as angulation during TMVR deployment can result in technical difficulties and imperfect results. Thus, the puncture site is determined preoperatively using 3D reconstruction of the CT scan, which will allow a near perpendicular direction of the delivery system in relation to the mitral annulus. The size of the purse-string is large, reflecting the size of the current delivery system. Difficulties can be encountered at the puncture site due to the thickness of the myocardium. The presence of an apical aneurysm, thrombus, or a thin, friable ventricle would be contraindications for this approach.
TS APPROACH (Figure 4B)
Percutaneous access through the femoral vein has been used for balloon mitral valvuloplasty for several decades and more recently also for transcatheter procedures such as MitraClip® (Abbott Vascular, Santa Clara, CA, USA) and valve-in-valve replacements in the mitral position. For most procedures, the tear/puncture of the interatrial septum can be left alone without any clinically relevant consequences. However, delivering a large TMVR device with a large delivery system will result in a much larger tear, which will need to be closed with a device11. Furthermore, the nature of the current delivery systems may not allow optimum manipulation for placement of the device in a perfect position, and can add to the complexity of an already complex procedure. This is supported by the fact that the majority of valve-in-valve procedures in the mitral position are performed through a TA approach12. It is, however, conceivable that, with improvements in the device and delivery system technology, this approach will become viable in future. At present, only two TMVR devices with human experience can be delivered transseptally, i.e., CardiAQ (Edwards Lifesciences, Irvine, CA, USA) and Caisson (Caisson Interventional [now LivaNova], Maple Grove, MN, USA).
TAt APPROACH (Figure 4C)
Delivery of a transcatheter mitral valve via transatrial access for a valve-in-valve or valve-in-ring device implantation has been described. The feasibility of this approach in the native mitral annulus has only been demonstrated in animal models13. Essentially, a small thoracotomy is performed on the right side and the left atrium or right superior pulmonary vein is accessed. After placing purse-strings, a short delivery system is used to implant the valve in the mitral position. Although this approach may allow better control of the implant due to proximity to the mitral valve and the antegrade nature of delivery, it still remains a surgical approach and coaxiality of the device is not always feasible.
TMVR DEVICES
We describe devices with first-in-man (FIM) experience and highlight some of the unique features, advantages and disadvantages of these devices.
CardiAQ valve system (Figure 5A, Table 1)
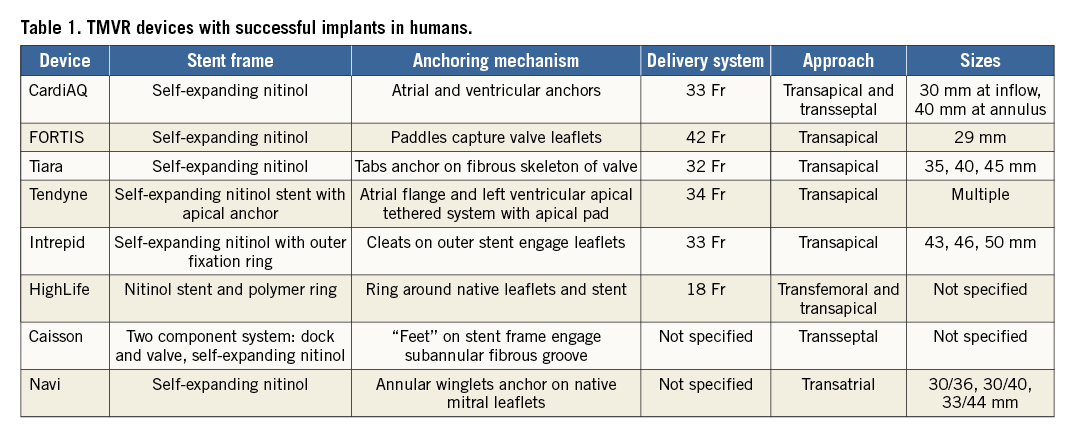
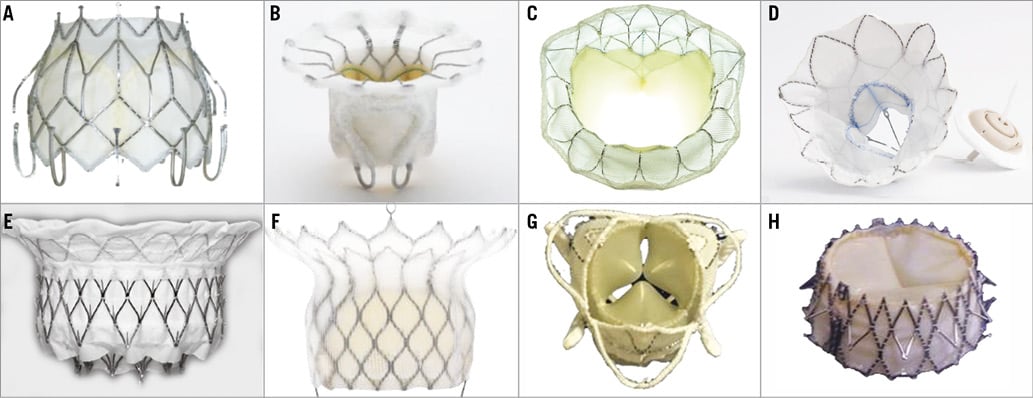
Figure 5. TMVR devices implanted in humans. A) CardiAQ valve system. B) FORTIS valve. C) Tiara. D) Tendyne valve. E) Intrepid (Medtronic). F) HighLife. G) Caisson. H) Navi Mitral Valved Stent.
– Stent frame: self-expanding nitinol frame, circular
– Leaflets: three leaflets made of bovine pericardium
– Anchoring mechanism: two sets of opposing anchors, atrial and ventricular. Ventricular anchors hook around the leaflets
– Suitable for native annulus size: 36 to 39.5 mm
– Delivery system: 33 Fr
– Approach: TA and TS
– Effect on LVOT: minimal as the device sits relatively high in the atrium
– Sizes: single 30 mm at the inflow and 40 mm at the annulus
– Clinical experience to date: the first implant was performed in 2012 with the first-generation CardiaAQ porcine bioprosthesis through a TS approach. From May 2014 to June 2015, the second generation CardiAQ bovine bioprosthesis was implanted in 10 patients. The TA approach was used in nine cases and the TS approach was used in one case14. The CardiaAQ valve has been implanted in 10 patients in a compassionate use protocol. The outcomes of three patients who underwent TMVR through the apex were published. Successful implant was achieved in all subjects. The thirty-day mortality rate was 33.3% without late mortality. Of the three patients, one patient died in hospital as a result of postoperative pneumonia14,15. The same group published the successful implant of the second generation of the CardiAQ valve through transfemoral-transseptal approach in one patient16
– Stage of development: feasibility trial
FORTIS™ (Edwards Lifesciences) (Figure 5B)
Stent frame: self-expanding nitinol frame, circular and cylindrical
Leaflets: three bovine pericardial leaflets symmetrical
Anchoring mechanism: the FORTIS TMV uses paddles located in the outflow of the central valve body allowing capture of the mitral leaflets
Suitable for native annulus size: 30 to 44 mm
Delivery system: 42 Fr
Approach: TA
Effect on LVOT: contraindicated in a small left ventricle
Sizes: single: 29 mm
Clinical experience to date: twenty patients have been treated with the FORTIS TMV17. In the USA, the results of only 13 patients performed outside trial protocol are available. Procedural success was obtained in 10 patients. Two patients required conversion to open surgery, one due to malposition and another due to chordal entanglement. One patient had partial migration of the valve and died on post-procedure day 4. The in-hospital mortality rate was 30.8% (4/15). Good results in abolishing MR were seen on the echocardiogram at the time of discharge. The 30-day mortality rate was 38.5%, as one patient died on day 15 post implant due to suspected valve thrombosis/endocarditis17
Stage of development: on hold/withdrawn
Tiara™ (Neovasc Inc., Richmond, BC, Canada) (Figure 5C)
Stent frame: self-expanding nitinol frame, D-shape
Leaflets: three asymmetric leaflets made of bovine pericardium
Anchoring mechanism: three tabs, two anterior and one posterior. The tabs help to secure the device by anchoring it against the fibrous skeleton of the mitral valve
Suitable for native annulus size: the A-P diameter is 30-34 mm, and the lateral diameter is 35-40 mm
Delivery system: 32 Fr
Approach: TA
Effect on LVOT: minimal as it is a D-shaped device
Sizes: multiple, although the only size available for investigational use is 35 mm. Other sizes (40 mm and 45 mm) are under development
Clinical experience to date: 19 patients have been treated with the Tiara valve. These patients were considered high-risk for mitral valve surgery. Three patients were reported to have malposition, with conversion to open surgery (16%). The remaining valves were successfully implanted and the 30-day echocardiogram evaluation showed no evidence of mitral regurgitation18. Thirty-day mortality was 16% (three patients). There was one case of late mortality on day 69 post implant despite successful implantation of the valve with abolishment of MR due to refractory end-stage heart failure19
Stage of development: feasibility trial, the TIARA-I study is actively enrolling at the present time
Tendyne/Lutter TMVR (Tendyne, Roseville, MN, USA) (Figure 5D)
Stent frame: two self-expanding nitinol stents. Outer stent is D-shaped
Leaflets: three symmetric leaflets made of porcine pericardium
Anchoring mechanism: atrial flange and left ventricular apical tethered system with apical pad
Suitable for native annulus size: 30 to 43 mm
Delivery system: 34 Fr
Approach: TA and TS (feasibility study for transseptal access route underway)
Sizes: multiple
Clinical experience to date: the first two patients who underwent a successful temporary implant of a Tendyne valve were operated in Paraguay under the International Organisation for Standardisation regulations20 (Muller D. Transcatheter mitral valve replacement: new valves and experiences. Presented at EuroPCR 2015, Paris, France). These two valves were explanted as per the protocol agreed and replaced with a surgical valve. A series of 30 patients was subsequently reported. All patients underwent a Tendyne valve implant via TA approach. The majority of patients (76%) treated had secondary MR. In 89% (26 patients) the ejection fraction (EF) was >30%. Two patients required device retrieval, one because satisfactory position of the implant was not achieved, and the second due to LVOT obstruction. Thirty-day mortality was 0%; information on late mortality is not available. One patient had haemolysis requiring transfusions and one patient had valve thrombosis. Overall results were impressive with no apical complications, improvement in functional class and abolition of MR20
Stage of development: feasibility trial
Intrepid™ (Medtronic, Minneapolis, MN, USA) (Figure 5E)
Stent frame: self-expanding nitinol frame which has a unique dual structure design consisting of a circular inner stent to house the valve and a conformable outer fixation ring to engage the mitral annular anatomy
Leaflets: trileaflet bovine pericardial valve
Anchoring mechanism: unlike other devices, the Intrepid valve is retained due to its unique interaction with the mitral annulus. The “cork effect” produced at the level of the annulus due to the variable stiffness of the stent frame is the primary mechanism for fixation. Small cleats on the outer stent also help by engaging with the mitral leaflets and promoting tissue ingrowth. The conformable outer stent engages the annulus, providing fixation and sealing while isolating the inner stent from the dynamic anatomy. The circular inner stent houses a 27 mm tricuspid bovine pericardial valve. The flexible brim aids imaging during delivery and subsequent healing21
Suitable for native annulus size: 30 to 42 mm (96% of screened patients)
Delivery system: 33 Fr
Approach: transapical
Effect on LVOT: minimal, as the stent is short
Sizes: 43 mm, 46 mm, and 50 mm outer diameters
Clinical experience to date: the latest data presented included 38 patients enrolled in the Intrepid TMVR early feasibility study with successful deployment in 36 out of 38 patients21. Thirty-day mortality was seven out of 38, with cause of death not related to the procedure in 3/7 and cause of death related to the procedure but not the device in the remaining four. There was one additional mortality between three and six months, which was not related to the procedure. Abolition of MR and improvement in the functional class were seen in all patients
Stage of development: feasibility trial
HighLife™ (HighLife SAS, Paris, France) (Figure 5F)
Stent frame: nitinol
Leaflets: glutaraldehyde cross-linked bovine pericardium
Anchoring mechanism: a ring placed around the native leaflets (subannular implant [SAI]) and a specifically designed stent with a groove placed inside the SAI. The SAI together with the native leaflets provide complete paravalvular sealing22
Suitable for native annulus size: not specified
Delivery system: first access is made to the femoral artery and an 18 Fr introducer sheath is positioned
Approach: transatrial and transfemoral approaches
Effect on LVOT: not specified
Sizes: not specified
Clinical experience to date: the single-centre early feasibility clinical trial started in Kiev (Ukraine). A first patient was treated successfully and discharged home at day seven23
Stage of development: feasibility trial enrolling
Caisson TMVR (Caisson Interventional) (Figure 5G)
Stent frame: Dacron panels for tissue ingrowth, D-shaped sealing cuff
Leaflets: porcine pericardium
Anchoring mechanism: unique “feet” on the outer stent frame provide anchoring by engaging with the subannular fibrous groove
Suitable for native annulus size: not specified
Delivery system: not specified
Approach: transseptal
Effect on LVOT: not specified
Sizes: not specified
Clinical experience to date: first-in-human PRELUDE, US early feasibility study
Stage of development: preclinical trials underway
Navi™ Mitral Valved Stent (NaviGate Cardiac Structures Inc., Lake Forest, CA, USA) (Figure 5H)
Stent frame: self-expanding nitinol stent
Leaflets: trileaflet, fabricated from bovine pericardium
Anchoring mechanism: self-expanding 21 mm height nitinol stent with a truncated cone configuration and annular winglets for anchoring the native mitral leaflets. Annular winglets are attached around the lower portion of the valve for secure anchoring23
Suitable for native annulus size: not specified
Delivery system: not specified
Approach: transatrial
Effect on LVOT: not specified
Sizes: 30/36, 30/40 and 33/44
Clinical experience to date: successfully implanted in two patients via a transatrial approach. Both patients had excellent valve performance without residual mitral regurgitation or LVOTO. The first patient showed significant improvement in functional class and freedom from hospitalisation at six months, but the second patient died within a week of the implant due to advanced heart failure
Stage of development: preclinical trial
Patient selection
As with any novel treatment, the early experience was in inoperable cases but, once the proof of concept had been established, the majority of the experience has been in the high-risk cohort. Both primary MR (25%) and secondary MR (75%) have been treated with TMVR. One of the factors determining enrolment has been experience and confidence in approved repair techniques such as MitraClip. Needless to say, only symptomatic MR patients have been treated.
As mentioned earlier, there are anatomic and physiological limitations for suitability for TMVR. These are confirmed with a transoesophageal echocardiogram (TEE) and a cardiac CT specific for the mitral valve. Echo assessment also looks at EF. Patients with EF <20% are not included in most feasibility studies as it is uncertain at this time if treatment in a “burnt out” ventricle is prognostically useful. TEE assessment is also critical as the procedure is performed under TEE guidance and it is important preoperatively to confirm that the views are optimal for the TMVR procedure.
Cardiac CT scan is the cornerstone of patient screening as detailed analysis is performed to confirm the suitability for the TMVR device with respect to size and implantability. Each TMVR device has device-specific criteria. Common to most devices are measurements of the mitral valve annulus. As the mitral valve shape and dimensions change during the cardiac cycle, multiple measurements are carried out throughout the cardiac cycle. Commissure to commissure distance, septolateral distance, circumference and area are measured. An oversize of 15-25% is applied to select the TVR size. Some devices need additional measurements such as length of the anterior and posterior leaflets in the case of the FORTIS TMVR device or degree of calcification in the annulus in the case of the Intrepid TMVR device. Similarly, CT analysis is used to analyse papillary muscle and chordae morphology, which is critical in devices such as Tiara, FORTIS, CardiAQ and HighLife. Accessory chordae, bifid or accessory papillary muscle may exclude the use of these devices.
As the majority of the devices are currently implanted through a transapical approach, it is important to make sure that the access site is suitable for the procedure. Thus, thinned out ventricles (<5 mm) are a contraindication for the TA approach. Patient selection will evolve hand in hand with increasing experience and will be partly dictated by the success and complication rate of the procedure.
Optimal medical management has been the cornerstone of treatment of heart failure associated with MR. It is appropriate to select patients who continue to be symptomatic despite optimal medical management or where optimal medical management is not desirable due to poor renal function. It is unclear at present whether reduction in MR is sufficient compared to elimination of MR. Increasing experience and possibly randomised trials may address this issue in future. Patient selection is based on “Heart Team” assessment of the patient but tends to fall into the high-risk category with the EuroSCORE or STS score.
Future direction
The mitral valve is undoubtedly the next frontier in structural valve disease after the success of TAVI. With reasonable intermediate results, data on long-term outcomes are needed to establish the safety, efficacy, and durability of transcatheter approaches to mitral valve repair and replacement. As with all new procedures, appropriate patient selection will be needed for optimal results. Determination of surgical risk, frailty, and comorbidities will play an important role in this selection. Furthermore, a better understanding of the anatomical and physiological factors affecting the implantability and outcomes associated with these devices is essential. Preprocedural planning, including multimodality CT and transoesophageal echocardiographic imaging, plays a critical role in device selection and procedural guidance. The need for device-specific anticoagulation will also be important. Anticoagulation strategy is still unclear and may play an important role in the success of TMVR, as the devices are large in size, covered with fabric, and placed in many patients with underlying atrial fibrillation. As in TAVI, for now the TA approach will probably remain the most common access site, but developments in the delivery system and device profile will definitely move this technology towards the less invasive TS approach. TMVR success will also depend on further improvements in mitral repair and replacement technologies.
Conflict of interest statement
V. Bapat is a consultant to Edwards Lifesciences, Medtronic Inc, Abbott, 4Tech and Boston Scientific. A. Patel has no conflicts of interest to declare.

