Abstract
Aims: With the recent developments in the field of transcatheter aortic valve replacement for aortic stenosis there has been a similar advance in the field of transcatheter mitral valve therapy for mitral regurgitation (MR). Both the anatomy of the mitral apparatus and the spectrum of pathology of MR are more complex than for aortic valve disease, and thus the development of MR therapies has been more complicated and less rapid.
Methods and results: The purpose of this review of recent literature is to provide a synopsis of the present technologies under development for percutaneous therapy for MR. Leaflet repair with MitraClip has accrued the largest human experience among the technologies that are under development, having been used to treat over 6,000 patients. MitraClip is currently being used in patients with functional MR and at high risk for conventional surgery. Coronary sinus, or indirect annuloplasty, has the next largest clinical experience, with several hundred patients treated in trials. Other MR therapy devices, including several direct annuloplasty approaches, mitral valve replacement prostheses, and chordal replacement devices, are still in the earlier phases of development.
Conclusions: The early technological advances have not only enhanced our understanding of the complex interplay of different components of the mitral valve apparatus but also promise continued refinement in our present modalities of treatment and improved clinical outcomes for future patients.
Introduction
Shortly after the first reports of percutaneous mitral and aortic valve therapies it seemed that the simplicity of coronary sinus annuloplasty would lead to rapid early development and adoption of this therapy, while the apparent complexity of percutaneous aortic valve replacement would result in a slower development pathway. One paper published in 2006 predicted that by the end of 2008 there would be over 2,000 patients treated with percutaneous mitral annuloplasty and 400 with transcatheter aortic valve replacement (TAVR)1. In fact, the opposite came to pass. This review will provide an update on the current status of catheter-based approaches for mitral regurgitation (MR) therapy and the future prospects and challenges in the field.
Percutaneous mitral leaflet repair with the MitraClip
Amongst the percutaneous MR treatment devices, the MitraClip (Abbott Vascular, Abbott Park, IL, USA) has, by far, amassed the largest experience both in trials and in practice (Figure 1). The progression from preclinical testing to a successfully completed phase 1 clinical trial, Endovascular Valve Edge-to-Edge Repair Study –EVEREST 1, led rapidly to a randomised comparison of the MitraClip with conventional surgery in EVEREST 22-6. The trial compared use of the mitral clip with conventional mitral valve surgical repair or replacement with a 2:1 randomisation. The basic guideline-defined indications for intervention for MR were used7. Patients with 3+ or 4+ MR and symptoms were included, regardless of functional or degenerative aetiology of MR. The primary efficacy endpoint was the composite of freedom from surgery for mitral valve dysfunction, 3+ or 4+ MR, and death at 12 months. The primary safety endpoint was defined as a composite of death, myocardial infarction, reoperation for failed mitral valve surgery, non-elective cardiovascular surgery for adverse events, renal failure, stroke, deep wound infection, prolonged mechanical ventilation, gastrointestinal complication requiring surgery, septicaemia, new onset permanent atrial fibrillation, and transfusion of two units or more of blood at 30 days.
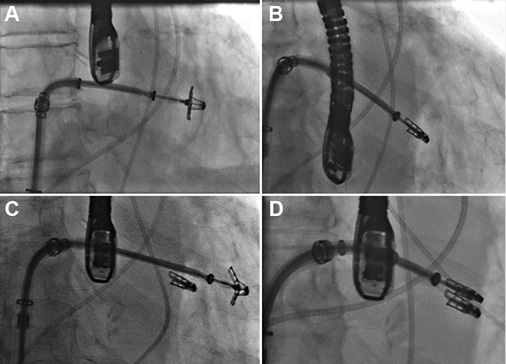
Figure 1. Fluoroscopic images of a MitraClip procedure. A) A MitraClip device has been passed across the mitral leaflets. B) The clip has been pulled back to grasp the leaflets. C) A second clip is passed adjacent to the first. Two clips are used in 40% of cases. D) The second clip has grasped the leaflets and been closed.
Two hundred and seventy-nine patients were randomised. MitraClip met the one-year efficacy endpoint for non-inferiority. The device group achieved the composite of freedom from death, surgery for mitral valve dysfunction, or 3+ or 4+ MR in 55% vs. 73% in the surgical group (p=0.0007) on an intention-to-treat analysis. The need for surgery after MitraClip therapy was responsible for the difference, 20% in the MitraClip group at 12 months vs. 2.2% for repeat surgery in the surgical patients. Importantly, death and MR grade 3+ to 4+ were not different in the device compared to the surgical group. Of the MitraClip recipients, 80% did not need surgery in the first 12 months, and from six months to three years the need for additional surgery was minimal in both groups.
Both percutaneous and surgical groups achieved meaningful clinical improvements. At one year, 98% of the MitraClip patients and 88% of the surgical patients were NYHA Class I/II. Both groups had significant reductions in LV volumes and dimensions and had quality of life improvements at 12 months.
A subgroup analysis of the randomised EVEREST 2 data showed that, in patients with functional rather than degenerative MR, older patients and those with poorer left ventricular function had results that were most comparable to surgery6. This observation, coupled with growing clinical experience, has led to the application of the MitraClip primarily in high-risk-for-surgery patients with functional MR (FMR). The outcomes in this group are characterised in the EVEREST High Risk Registry8. Seventy-eight patients with moderate to severe MR and an estimated surgical mortality risk of >12 percent (measured with the Society of Thoracic Surgery calculator or based on assessment by a surgeon) were enrolled. The anatomic mitral valve selection criteria were identical to the randomised trial, yielding a population clearly not usually treated with surgery. The patients were elderly, many having had previous cardiac surgery (62%), moderate to severe renal disease (23%), COPD (35%), and previous myocardial infarction (56%). Most had FMR9. The mean STS risk score was over 12%.
Significant improvements were seen in LV dimensions, NYHA Class and quality of life scores. Annual hospitalisations for heart failure were decreased by almost half from baseline. Overall 30-day mortality in the high-risk group and control groups were similar, (7.7% and 8.3%, respectively) indicating no “penalty” for performing the procedure in these very sick patients. After one year, survival was improved in the MitraClip-treated patients compared to the control group (76.4% vs. 55.3%, p=0.047).
The consistent experience with MitraClip for these very sick, high-risk patients has been positive. These patients have never had an option for treatment in the past, and thus the good clinical outcomes after edge-to-edge repair are novel. The degree of clinical improvement is often discordant with the amount of residual MR. Many patients have persistent 2+ or 3+ MR, and nonetheless have favourable LV chamber remodelling and symptomatic improvement. The rapid adoption of this procedure in Europe is consistent with the EVEREST High Risk Registry experience. Several thousand patients have been treated commercially, with most having a profile like the EVEREST High Risk Registry patients. More than two thirds have FMR and most are high risk (Table 1, Figure 2). Patient selection in the commercial experience has been extended beyond the narrow EVEREST selection criteria. In the EVEREST trials, there was strict adherence to inclusion of several echocardiographic leaflet anatomic features and a lower LVEF cut-off of 25%6. The “real world” use of the device has employed a more liberal selection criterion with acute safety and six-month efficacy that is similar to the EVEREST trial outcomes9-11. Another group with MR and particularly poor clinical outcomes are non-responders to cardiac resynchronisation therapy. Even these patients have had favourable LV remodelling and increased LVEF a year after MitraClip implantation12.
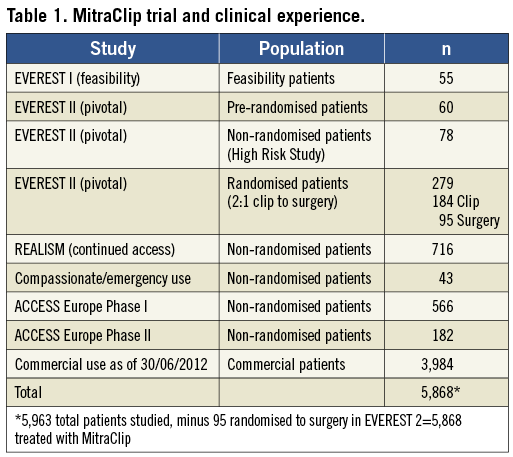
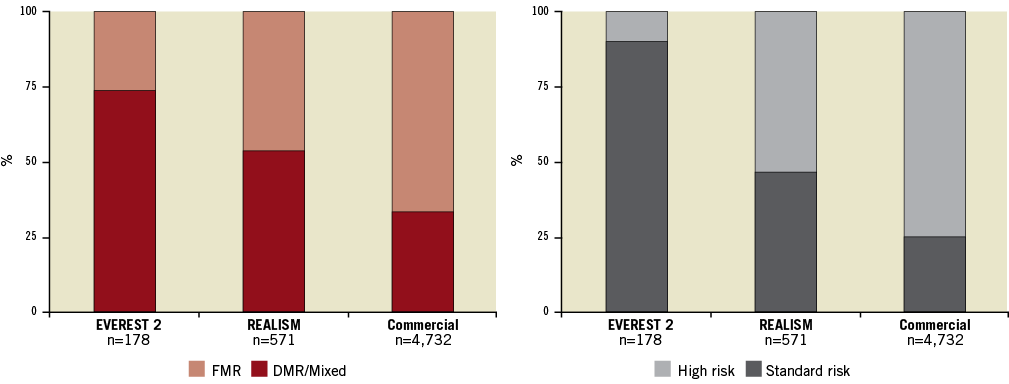
Figure 2. Left: the randomised EVEREST 2 trial primarily treated patients with degenerative mitral regurgitation (DMR), who were standard operative risk and were representative of patients who have traditionally been treated with surgery. The proportion of patients with functional mitral regurgitation (FMR) has increased steadily as trial experience has been gained. Right: the risk profile of patients selected for MitraClip has steadily increased. In “real world” practice currently the vast majority of patients are high risk for surgery. The patients analysed in the EVEREST high-risk registry represented a high-risk subset taken from the REALISM registry.
The results from MitraClip have improved significantly since the inception of the procedure13. The rates of successful clip implantation and MR reduction have improved and now exceed 95%. In the early experience a single mitral leaflet would detach from the MitraClip in almost 10% of cases4. Importantly, this problem of partial leaflet detachment is not associated with device embolisation. MR does recur, and this problem accounted for about half the early crossovers from MitraClip to surgery in the randomised EVEREST 2 trial6. The rate of partial leaflet detachment has been decreased to less than 2% in our recent experience13. This is the result of careful echocardiographic assessment of leaflet insertion into the clip arms during the procedure14.
Many questions remain unanswered regarding MitraClip therapy. While there are as yet no randomised trials, there is little controversy regarding the use of MitraClip for poor surgical candidates, particularly with FMR. Plans for randomised trials of MitraClip compared with medical therapy in both Europe (Randomized Study of the MitraClip Device in Heart Failure Patients With Clinically Significant Functional Mitral Regurgitation –RESHAPE-HF) and the United States (Clinical Outcomes Assessment of the MitraClip Percutaneous Therapy for High Surgical Risk Patients –COAPT) are underway to define further its use in the high-risk subset. Uncertainty about the impact of residual MR and the durability of MR reduction after MitraClip limit the further expansion of the therapy into patient groups which are currently candidates for surgery, despite the lack of randomised trials demonstrating a clear benefit of surgery in FMR.
Coronary sinus annuloplasty
While several thousand patients have been treated with MitraClip, only several hundred have had implants of all the other mitral devices combined. After MitraClip, the next largest experience is with the Carillon® device (Cardiac Dimensions, Inc., Kirkland, WA, USA), a coronary sinus (CS) implant that achieves an indirect annuloplasty15. Several prior devices in the indirect annuloplasty category have fallen by the wayside and are no longer under development16. One of the unanticipated problems for CS implants is device fracture16. The torsional forces exerted by the CS are substantial. An initial trial of the Carillon showed efficacy for MR reduction, but had wire fracture in several cases17. These occurred after only a few months. Importantly, wire fractures were not associated with clinical complications. Improvements in the Carillon appear to have solved the problem of wire fracture. Bench testing is now able to reproduce wire fracture in first-generation devices, while the newest version of the device does not fracture under the same stresses.
The Carillon device is a fixed-length double-anchor implant with mirror-image hoop-shaped helical anchors (Figure 3). The CS is cannulated from the internal jugular venous approach with a 9 Fr delivery catheter. The distal anchor is deployed deep in the CS. Traction is placed on the delivery system and guided by fluoroscopy and TEE to plicate the annulus. After plication is optimised, the proximal anchor is deployed near the CS ostium. Because the circumflex artery crosses under the CS, angiography is performed to assess coronary flow. If the circumflex is compressed the device can be recaptured.
A recent report documented the outcome in 53 patients treated with the Carillon18. The implant success rate was two thirds. The remainder of the patients either had CS anatomy not suitable for the device, or transient circumflex coronary compression which precluded leaving the device in place. Among the remainder there were improvements in MR severity, LV volumes, and clinical parameters such as quality of life after one year. Clinical improvements persisted for 24 months. The complication rate was <2%. This device has CE approval in Europe and larger trials will be conducted to compare the Carillon to medical therapy in patients with severe MR and heart failure.
Direct annuloplasty
Several devices that achieve a direct annuloplasty are in early development. Small numbers of patients have been treated and no completed phase 1 trials have been reported. Direct annuloplasty has the potential to mimic surgical annuloplasty more closely. The problem of circumflex coronary compression that occurs with the CS approach is also solved by direct annuloplasty. There are currently several direct annuloplasty devices under development. The Mitralign system (Mitralign, Tewksbury, MA, USA) and the Guided Delivery Systems Accucinch® device (Guided Delivery Systems Inc., Santa Clara, CA, USA) both use retrograde access to the LV to deliver devices directly into the mitral annulus. For the Mitralign system, wires are passed from the LV side through the mitral annulus into the left atrial side of the annulus. Two pairs of pledgets are passed over the wires as anchors and connected with a draw-string. Tension on the string draws the pledgets together to shorten the mitral annular circumference (Figure 4). The mitral annulus circumference can be shortened by as much as 3 cm. For the Accucinch device, a specially designed delivery catheter is passed retrogradely from the LV side, under the posterior leaflet, and around the annulus (Figure 5). Through a series of evenly spaced openings in the delivery catheter, nitinol anchors can be placed in the annulus. These anchors are connected with a cord. Tension on the cord draws the anchors together with reduction of the mitral circumference. This device has been used surgically and in early human experience has demonstrated feasibility. A more recent entrant in the category of direct annuloplasty utilises transseptal puncture with delivery of the device to the atrial side of the mitral annulus, in contrast to the previously described retrograde transaortic approaches. An implant is delivered from trigone to trigone, resulting in an incomplete ring (Figure 6). It is anchored with nitinol screws directly into the annulus. This device has been implanted surgically, and a percutaneous method is fairly well developed. Early human experience is pending. Yet another method for catheter-based annuloplasty uses radiofrequency energy. This system has been developed in a bench model and in a surgical animal model. Radiofrequency energy is used to heat and shrink the collagen in the mitral annulus, with a resultant decrease in mitral annular circumference19.
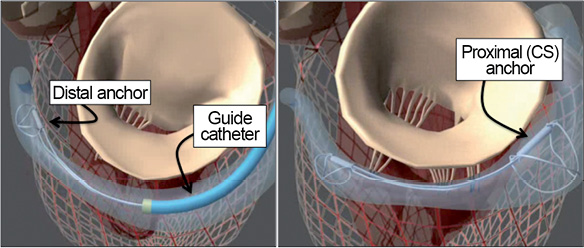
Figure 3. Coronary sinus (CS) annuloplasty with the Cardiac Dimensions Carillon® device. A guide catheter is engaged in the CS and the device is anchored in the distal CS or great cardiac vein. The guide and device are pulled back together to cinch the annulus, and the proximal anchor is then released in the CS ostium.
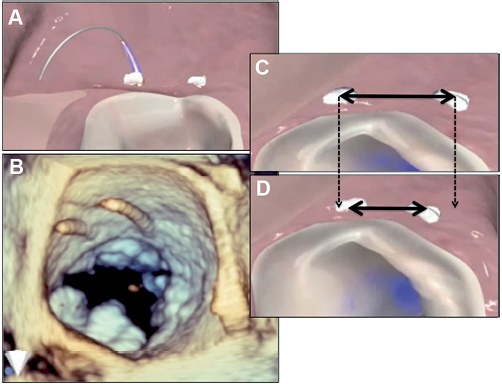
Figure 4. Direct annuloplasty with the Mitralign system. A pair of pledgets is placed in the mitral annulus near one commissure and are drawn together to shorten the mitral annular circumference. A second pair of pledgets is placed adjacent to the other commissure. A and B show the wires used for passage of the pledgets. C and D show shortening of the tissue between the pledgets.
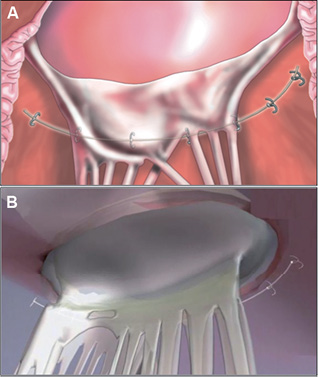
Figure 5. The Guided Delivery Systems Accucinch® places a delivery catheter under the posterior leaflet and then delivers anchors into the annulus. The anchors are drawn together with a cord or tether. There is some remodelling of the base of the LV as well as the annulus.
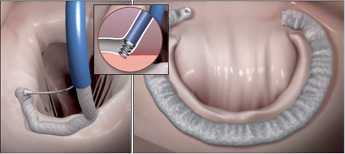
Figure 6. The Valtech Cardioband (Valtech Cardio, Or Yehuda, Israel) is placed from a transseptal approach and anchored directly into the mitral annulus.
One normal beating heart surgical approach for mitral annuloplasty combined with left ventricular reshaping defies categorisation. The Myocor® approach (Myocor, Inc., Maple Grove, MN, USA) placed specially designed pads on the external aspect of the anterior and posterior surfaces of the beating heart during coronary bypass operations in patients with ischaemic MR. The pads were connected by a tether that ran directly through the LV chamber. This resulted in compression of the septal-lateral annular dimension, and immediate remodelling of the left ventricular chamber. This approach was tested in a randomised trial compared to conventional annuloplasty20. While there was less reduction of MR with the Myocor® device than with surgery, survival was better. Two completely percutaneous transpericardial procedures were successfully performed with this device. Unfortunately the company lost funding and there has been no further development. The concept is attractive as this is the first device to accomplish annuloplasty successfully and at the same time to remodel the LV chamber.
Another surgical technique being translated into a percutaneous approach is chordal replacement. Beating heart surgical devices for chordal replacement have been used in patients, and efforts are underway to translate these into percutaneous devices21.
Transcatheter mitral valve replacement
TAVR for aortic stenosis has created an expectation that a stent-mounted bioprosthetic valve replacement device may also be applicable in the mitral position. There are several unique challenges facing percutaneous mitral replacement. The mitral orifice is substantially larger than the aortic annulus. Calcification of the aortic leaflets provides anchoring in that position, but for the mitral valve anchoring is probably the greatest problem. Percutaneous mitral valve replacement for pre-existing bioprosthetic valves has been accomplished using already approved TAVR devices. Similarly, approved TAPVR devices have been implanted successfully in previously placed mitral annuloplasty surgical rings. Both of these situations provide a landing zone for a conventional stent valve. To treat the native mitral valve, some dedicated anchoring system will be required. At the time of writing only a single patient has been treated with a dedicated percutaneous mitral valve replacement device. This was a compassionate use situation, and no phase 1 trials have been initiated to date22.
Future perspective
The development pathway for percutaneous mitral repair has taken longer than that for TAVR. The complexity of the mitral apparatus, widely varying aetiologies for MR and a wide range of surgical procedures that are models for the development of less invasive approaches explain this difference in development timelines. Even if predictable and effective percutaneous mitral replacement devices are developed, there will probably still be applications for percutaneous repair devices. We are at the beginning of the percutaneous mitral therapy effort, and it will take a great deal of additional work and clinical research to define practice in this field.
Conflict of interest statement
T. Feldman is a consultant to Abbott, Boston Scientific and Edwards Lifesciences. O. Ali has no conflict of interest to declare.

